* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Normocytic Anemia
Ulcerative colitis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Molecular mimicry wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Hookworm infection wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Autoimmunity wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
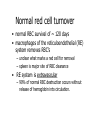
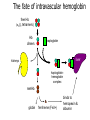
Normocytic Anemia David Lee, MD, FRCPC Normocytic anemia • a heterogenous group of anemias • normocytosis implies normal DNA metabolism and hemoglobin synthesis • no common pathophysiology Approach to anemia anemia check MCV MCV < 80 microcytic anemia MCV 80 - 100 normocytic anemia MCV > 100 macrocytic anemia Approach to normocytic anemia normocytic anemia Is there increased red cell production? check reticulocyte count increased Is there evidence of hemolysis? yes hemolytic anemia normal or decreased Is there evidence of: - renal failure - endocrine failure - chronic inflammation anemia of renal failure anemia of endocrine failure anemia of chronic disease no recent bleed If not, then consider a primary marrow problem (MDS, MM, infiltration…) bone marrow investigation Approach to normocytic anemia normocytic anemia Is there increased red cell production? check reticulocyte count increased Is there evidence of hemolysis? yes hemolytic anemia normal or decreased Is there evidence of: - renal failure - endocrine failure - chronic inflammation anemia of renal failure anemia of endocrine failure anemia of chronic disease no recent bleed If not, then consider a primary marrow problem (MDS, MM, infiltration…) bone marrow investigation Anemia of chronic disease • Anemia due to cytokines IL-1, TNF-a, TGF-b associated with chronic inflammation – rheumatoid arthritis – systemic lupus erythematosis – inflammatory bowel disease – chronic infections: osteomyelitis, TB Anemia of chronic disease • Mechanisms – defective iron utilization • iron is present, but cannot be utilized by erythroid precursors – impaired response to erythropoietin – ?blunted increase in erythropoietin in response to anemia Anemia of chronic disease • usually normocytic, but 25% are microcytic • usually mild to moderate anemia • anemia of chronic disease is a diagnosis of exclusion • bone marrow often needed to rule out other causes of anemia Iron studies in IDA and ACD Test IDA ACD serum iron low low TIBC high transf. sat. low normal or low low serum ferritin marrow iron low absent normal or increased normal or increased Anemia of chronic disease • Treatment – treat the underlying cause – erythropoietin can be effective, but is expensive Anemia of chronic renal failure • Mechanism: – mainly due to reduced production of erythropoietin by diseased kidneys – also iron or folate deficiency, chronic inflammation, shortened red cell survival • Treatment – erythropoietin thrice weekly – dialysis Anemia of endocrine failure • Uncommon cause of anemia, but correctable – hypothyroidism – hypogonadism – pan-hypopituitarism Approach to normocytic anemia normocytic anemia Is there increased red cell production? check reticulocyte count increased Is there evidence of hemolysis? yes hemolytic anemia normal or decreased Is there evidence of: - renal failure - endocrine failure - chronic inflammation anemia of renal failure anemia of endocrine failure anemia of chronic disease no recent bleed Pure red cell aplasia If not, then consider a primary marrow problem (MDS, MM, infiltration…) bone marrow investigation Pure red cell aplasia • Very rare cause of normocytic anemia • immune-mediated destruction of red cell precursors in the marrow • reticulocyte count is low • treatment: immune suppression Approach to normocytic anemia normocytic anemia Is there increased red cell production? check reticulocyte count increased Is there evidence of hemolysis? yes hemolytic anemia normal or decreased Is there evidence of: - renal failure - endocrine failure - chronic inflammation anemia of renal failure anemia of endocrine failure anemia of chronic disease no recent bleed If not, then consider a primary marrow problem (MDS, MM, infiltration…) bone marrow investigation Hemolytic anemia • Anemia due to increased rate of RBC destruction • anemia occurs when rate of destruction exceeds production Normal red cell turnover • normal RBC survival of ~ 120 days • macrophages of the reticuloendothelial (RE) system removes RBC’s – unclear what marks a red cell for removal – spleen is major site of RBC clearance • RE system is extravascular – 90% of normal RBC destruction occurs without release of hemoglobin into circulation. The fate of intravascular hemoglobin free Hb (a2b2 tetramers) Hb dimers haptoglobin liver kidneys haptoglobinhemoglobin complex metHb globin ferriheme (Fe3+) binds to hemopexin & albumin Extravascular vs Intravascular hemolysis Test Extravascular Hemolysis Intravascular Hemolysis LD bilirubin N to absent absent hemoglobinuria absent present free Hb in plasma absent present urine hemosiderin absent present haptoglobin Causes of intravascular hemolysis – Mechanical • prosthetic heart valve, tight AS • march & bongo drummer’s hemoglobinuria – Microangiopathic • DIC, TTP, HUS – Immunological • acute hemolytic transfusion reaction, PNH – Infection • malaria • Clostridium welchii sepsis – Enzymopathy • severe G6PD deficiency Is there hemolysis? • Look for 3 lines of evidence: – 1. Damaged red cells on the blood film – spherocytes (immune hemolysis, HS) – red cell fragments (microangiopathic anemias) – 2. Marrow response to hemolysis – polychromasia on blood film – reticulocytosis – erythroid hyperplasia in marrow – 3. Biochemical evidence of RBC destruction – increased unconjugated bilirubin – increased lactate dehydrogenase – decreased/absent haptoglobin Approach to normocytic anemia normocytic anemia Is there increased red cell production? check reticulocyte count increased Is there evidence of hemolysis? yes hemolytic anemia normal or decreased Is there evidence of: - renal failure - endocrine failure - chronic inflammation anemia of renal failure anemia of endocrine failure anemia of chronic disease no recent bleed If not, then consider a primary marrow problem (MDS, MM, infiltration…) bone marrow investigation An approach to hemolytic anemia Hemolytic anemia Immune • Autoimmune • Alloimmune Non-immune Congenital Acquired Defects of: • RBC membrane/ skeleton • Infections sepsis malaria (eg. Hereditary spherocytosis) • Drug-induced (other causes of immune hemolysis are rare) • Mechanical • RBC enzymes (eg. G6PD deficiency) • Hemoglobin prosthetic heart valve microangiopathic HA An approach to hemolytic anemia Hemolytic anemia Immune • Autoimmune • Alloimmune Non-immune Congenital Acquired Defects of: • RBC membrane/ skeleton • Infections sepsis malaria (eg. Hereditary spherocytosis) • Drug-induced (other causes of immune hemolysis are rare) • Mechanical • RBC enzymes (eg. G6PD deficiency) • Hemoglobin prosthetic heart valve microangiopathic HA Immune hemolysis • most frequent cause of hemolysis • due to IgG or complement on red cells – tags the red cell for phagocytosis – spherocytes if incomplete phagocytosis – lysis of RBC occurs if complement cascade goes to completion Autoimmune hemolysis • Most common type of immune hemolysis • primary (idiopathic) • secondary – autoimmune hemolysis secondary to: • autoimmune condition (such as SLE) • infection • lymphoma or CLL Diagnosis of immune hemolytic anemia – 1. Direct Antiglobulin Test (DAT or direct Coomb’s test) – detects IgG or complement on patient’s red cells – the vast majority of patients with active immune hemolysis will have a positive DAT. – 2. Indirect Antiglobulin Test (IAT, indirect Coomb’s test) – detects antibody in patient’s serum against red cell antigens – A positive IAT does not necessarily mean hemolysis is occurring It may simply mean allo-immunization due to previous exposure to “foreign” red cell antigens (past pregnancy or transfusion). – 3. Peripheral Blood Film: spherocytes Treatment of autoimmune hemolysis • treat the underlying cause, if there is one • stop suspect drugs if possible • prednisone • transfuse RBC’s, if needed An approach to hemolytic anemia Hemolytic anemia Immune • Autoimmune • Alloimmune Non-immune Congenital Acquired Defects of: • RBC membrane/ skeleton • Infections sepsis malaria (eg. Hereditary spherocytosis) • Drug-induced (other causes of immune hemolysis are rare) • Mechanical • RBC enzymes (eg. G6PD deficiency) • Hemoglobin prosthetic heart valve microangiopathic HA Hereditary spherocytosis • most common inherited red cell membrane disorder • 1/5000 in northern European populations • autosomal dominant • caused by mutations in the genes that encode RBC membrane cytoskeleton proteins. Normal membrane cytoskeleton Hereditary spherocytosis loss of membrane = loss of SA = loss of deformability = increased splenic clearance Hereditary spherocytosis • Spherocytes are cleared by the spleen more rapidly – lack of deformability means they cannot squeeze through the sieve-like slits of the spleen. Hereditary spherocytosis • Clinical features: – clinical severity varies – most have mild to moderate anemia – splenomegaly, cholelithiasis, jaundice may occur • Laboratory features – hemolytic anemia with spherocytes – osmotic fragility test – negative DAT Hereditary spherocytosis • Treatment – most patients do not need treatment – splenectomy – counsel patient and family about inheritance An approach to hemolytic anemia Hemolytic anemia Immune • Autoimmune • Alloimmune Non-immune Congenital Acquired Defects of: • RBC membrane/ skeleton • Infections sepsis malaria (eg. Hereditary spherocytosis) • Drug-induced (other causes of immune hemolysis are rare) • Mechanical • RBC enzymes (eg. G6PD deficiency) • Hemoglobin prosthetic heart valve microangiopathic HA G6PD deficiency • Most common inherited red cell enzymopathy – up to 10% of those with African and Mediterranean descent • X-linked • hemolysis is due to increased oxidative damage to red cells G6PD deficiency • clinical severity highly variable – Most experience little or no anemia unless exposed to precipitating event or drug – precipitants: • infections • sulfa, primaquine, dapsone • fava beans G6PD deficiency • Laboratory diagnosis – bite cells – Heinz bodies – measure G6PD level • Treatment – supportive – avoid precipitants – counsel patient/family Anemia cases 1. A 45 year old woman presents to your office with a 2 week history of increasing fatigue and jaundice. No hepatomegaly or splenomegaly on examination. Hb MCV RDW WBC Platelets 85 97.5 19.8 13.4 457 (120-150) (80-96) (11.5-14.5) (4 - 10) (150-450) DAT IAT positive positive LD T bili direct bili serum haptoglobin Reticulocytes Peripheral blood smear: 301 (94-172) 53 (0-17) 2 absent 357 (18-94) some spherocytes g/L fL x 10 9/L x 10 9/L U/L mol/L x 10 9/L 2. You are the ER doc in a remote town, 2 hours from a tertiary care hospital. At 2300h, a 57 year old man is brought to the ER by his wife because of acute confusion and disorientation. She thought he had the “flu” for the past 4 days because he has had a fever, headache, and was feeling unusually tired. He has been on no medications and he had previously been well. Hb MCV WBC plt retics blood smear creatinine PT PTT 70 g/L 98 fL 14.8 x 10 9/L 76 x 109/L 267 x 10 9/L RBC fragments polychromasia thrombocytopenia neutrophilia with left shift 97 mol/L normal normal 3. A 30 year old woman with a 1 year history of rheumatoid arthritis, involving hands, wrists, On NSAIDS. ESR Hb MCV RDW WBC Plt Retics 57 mm/hr 108 g/L 80 fL 18.5 8.2 x 109/L 317 x 10 9/L 89 x 109/L 4. A previously healthy 67 year old man with recurrent pneumonia. Hb 103 MCV 98 WBC 3.9 Plt 153 Retics 56 Peripheral blood smear: rouleaux What next? urea creatinine normal LD 216 Coombs neg bili normal serum protein electrophoresis: IgG kappa monoclonal protein with depression of IgA, IgM and polyclonal IgG. Bone marrow investigation: excessive number of plasma cells Skeletal survey: numerous lytic lesions in the axial skeleton and cranium. This is case 3 from the iron lecture Case 3. A 72 year old woman with a history of rheumatoid arthritis is found to have a hemoglobin of 97 g/L (120-160) and an MCV of 79 fL (80-100). The white blood cell and platelet counts are normal. Review of the peripheral blood smear does not show much abnormality. She takes ibuprofen twice a day for her joint symptoms. 1. What is the differential diagnosis for her microcytic anemia? 2. What other items on history and physical would be helpful? 3. What other investigations are indicated?