* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Professor Rounds LSU NEUROLOGY
Survey
Document related concepts
Transcript
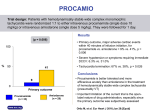
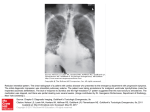
Professor Rounds LSU NEUROLOGY Raisa C. Martínez, PGY-2 29.Nov.12 • Hospital: Ochsner-Kenner Medical Center Admission Date: 10/11/12 Discharge Date: 10/22/12 • Staff Neurologist: Dr. Barton & Dr. Mader • Fellows: Dr. Obih & Dr. Mukardamwala Chief Complaint “My knees are weak.” History of Present Illness 53 yo. Caucasian, R-handed, male with PMHx of SHF, HTN, HPLD, DM2, A-fib, CAD, and COPD who presented to the hospital complaining of progressive weakness in his lower extremities bilaterally. Patient reported the symptoms developed approximately 3 weeks prior to admission, yet according to family members, these symptoms had begun to develop 2 months prior. Weakness developed in both extremities simultaneously and is not ascending in nature. There is also weakness in the upper extremities bilaterally, specifically the shoulders, which began shortly after the leg onset. He endorses that the lower extremity weakness is significantly worse than the upper extremities. Patient cannot climb the stairs of his house, and now is only able to walk with a shuffling gait. Denies any range of motion (ROM) limitations, but does take longer to do activities of daily living (ADL’s). • Patient cannot get up from a chair without assistance. • Denies sensory episodes of numbness, tingling, burning or cramping. • Denies pain, tremors, twitching, swelling, redness, or tenderness in muscles or joints. • Denies backaches, arthritis, or musculoskeletal trauma. Review of Systems: (+) • Gen: Unintentional weight loss (15 lbs in 8 wks.), decreased appetite, decreased energy, mild jaundice • CV: lower extremity edema • Pulm: Shortness of breath (chronic problem, yet now requiring O2) • GI: Constipation • Neuro: decreased/abnormal taste • Heme: Easy bruising, R shoulder/back hematoma Review of Systems: (-) • • • • • • • • • • • Gen: No fevers, chills or night sweats, no trauma Eyes: No changes in vision, photophobia or inflammation ENT: No dysphagia, epistaxis or tinnitus CV: No palpitations, chest pain, or claudication, or syncope Pulm: No cough with or without sputum, no paroxysmal nocturnal dyspnea, no orthopnea Endocrine: no heat/cold intolerance, no increased thirst, no hair loss GI: No N/V, abdominal pain, distension, changes in stool color or caliber GU: No dysuria, flank pain, hematuria, changes in frequency, or erectile dysfunction Neuro: No HA, dizziness, lightheadedness, LOC, AMS MSK: No arthralgias, no myalgias Skin: No rash, insect bite or soft tissue infections Past Medical History & Chart Check • Conditions: HTN, CAD, SHF (EF 30%), Afib, DM2, HLPD, COPD, GERD • Allergies: NKDA/NKFD • Surgeries: CABG (‘95 & ‘96) AICD (2003) Cholecystectomy • Family Hx: Mother (75) CAD Father (72) CAD, DM2, HTN No hx. of nerve/muscle disease • Social Hx: Smoke: 2pck/d x 35 years, quit 1 month prior EtOH: social events Illicit drugs: none OTC: none Herbs/Remedies: none Lives with mother • Recent Hospitalizations: 9/30/12 for Coumadin toxicity and right shoulder hematoma. Coumadin stopped … • Medications: Spiriva 18 mcg Coreg 25 mg bid Losartan 50 mg ASA 81 mg Plavix 75 mg Nexium 40 mg Amiodarone 100 mg Simvastatin 40 mg Ultram 50 mg PRN Folic acid 800mg Lasix 40 mg Physical Exam • GENERAL: AAO x3, moderately obese, no apparent distress. • HEENT: atraumatic,+ scleral icterus, no thyromegaly or cervical lymphadenopathy. Supple neck. • CARDIOVASCULAR: Irregularly regular rate and rhythm. No murmurs,rubs, or gallops. • RESPIRATORY: Minimal bibasilar crackles, poor breath sounds. • ABDOMEN: Protuberant, NT/ND,+BS, no hepato-splenomegaly. • EXTREM: + 3 edema bilateral LE, 1+ pulses LE, 2+ UE, Right LE muscle wasting • Skin: + mild jaundice, R shoulder hematoma. No rash, petechiae, or ulcers Neurologic Exam • Orientation Person, Place, Time • Memory Remote & recent intact • Language • Cranial Nerves: No aphasia • Fund of Knowledge Appropriate Aware of current events • Concentration Appropriate I: Intact (coffee & alcohol swabs) II:25/20 bilateral, VF intact II, III: PERRLA II,IV,VI: EOMI V1-3: intact bilaterally, clenches and grinds teeth, +corneal VII: symmetric facial expression ; abnormal taste VIII: AC>BC, no lateralization IX, X: uvula midline, symmetric palate elevation, + gag XI: TPZ 5/5 ; SCM 5/5 XII:tongue midline Motor Shoulder abd RIGHT 3/5 LEFT 3/5 Shoulder add Upper arm flex Upper arm ext Wrist flex 3/5 4/5 4/5 5/5 3/5 4/5 4/5 5/5 Wrist ext 5/5 5/5 Grasp Finger Spread Hip Flex Thigh abd Thigh add 5/5 5/5 3/5 4/5 4/5 5/5 5/5 3/5 4/5 4/5 Leg flex Leg ext Foot dorsifl ex Foot inversion Sole cupping Toe ext Great toe ext 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 5/5 … • DTR’s +2 Biceps, Triceps, Brachioradialis bilateral +1 Patellar bilateral 0 Achilles bilateral • Babinski: Absent • Coordination/Balance No dysmetria, no tremor, no Romberg • Gait Wide based, shuffling gait Extra steps to turn around Unable to get up from chair with arms crossed • Sensory Decreased pinprick bilateral plantar surfaces Otherwise, intact to light touch, pinprick, vibration, temperature and joint-position throughout Clinical Approach Differential Diagnosis • • • • Onset and Duration: Hyperacute (24-72 hours) Acute (< 1 month) Subacute (1 - 6 months) Chronic (> 6 months) Anatomic Classification: Fiber type: Motor vs sensory Somatic vs autonomic Fiber size Distribution Symmetry Length-dependent Axonopathy vs myelinopathy (?) Laboratory Work-Up • Hematology: • Chemistry: 95.8 134 105 51 103 4.8 20 1.4 Ca = 8.3 Mg = 2.6 Phos = 3.8 TPr Alb Tbi AST ALT AkP 6.9 2.3 1.3 39 8 82 11.2 10.8 226 18.5 33.5 Iron = 27 Ferritin = 427 Folate = 9.8 Vit B12 = 451 HgA1C = 5.4% FT4 = 1.14 TSH = 11.6 Lactic acid = 1.4 INR = 2.3 PT = 24.7 PTT = 42.7 CRP = 127 ESR = 42 LDH = 153 Aldo = 3.3 CPK = 12 … • CSF: Clear, 12 mL total Gluc = 73 Prot = 29 LDH = 16 Wbc = 0 Rbc = 0 Cultures : No organisms Arbovirus Panel : negative • Autoimmune: ANA: (-) RF: (-) Thyroperoxidase: (-) Thyroglobulin: 8.3 • Infectious RPR : (-) Hep Panel: (-) HIV: (-) Neuro-imaging Head CT • Remote Left posterior frontal lobe and Right frontal lobe lacunar infarcts Cervical/Thoracic/Lumbar CT • Cervical spondylosis C3-C4 • Mild thoracic and lumbar spondylosis Electrophysiology Sensory Nerve Conduction: Onset Latency Peak Latency Amp Segment Latency Diff Distance Conduction Velocity Sural R. NR NR NR NR NR NR NR Sural L. NR NR NR NR NR NR NR Median L. Digit I 4.3 ms 5.5 11µV Wrist digit 4.3 130mm 31m/s (50) Digit II 5.0 ms 5.8 ms (3.5) 6µV (20) Wrist-digit 5.0 ms 160mm 32m/s (50) Digit III NR NR NR Wrist-digit NR NR NR Ulnar L. Digit V NR NR NR NR NR NR NR Nerve/Site Electrophysiology Motor Nerve Conduction: Nerve/Site Latency Amplitude Segment Latency Diff Distance Conduction Velocity Peroneal L. NR NR NR NR NR NR 5.3 (6.7) 19.1 3.5mV (4) 2.3mV Ankle-Pop 13.8ms 365mm 26ms (40) NR NR NR NR NR Tibial R. Ankle Popliteal Peroneal R. Ankle Poplieal Tibial L Ankle Popliteal Ankle-Fib 6.5 ms (5.8) 0.6mV (4) Ankle -Pop Median L Wrist Elbow 5.6ms (4.4) 11.3 ms 1.6mV (4) 1.3mV Wrist-Elbow 5.7 ms 225 mm 39 ms (50) Ulnar L. Wrist Below elbow Above elbow 3.9 ms (4.5) 9.3 ms 12.1 ms 1.2 mV (7) 0.9mV 1.0mV Wrist-below Elbow Below Elbow-Above Elbow 5.4 ms 2.8 ms 225 mm 100 mm 42 m/s (50) 36 m/s Electrophysiology F-Wave Studies: Nerve Tibial R. Tibial L Median L Ulnar M-latency 9.1 16 6.9 4.6 F-latency 0.8 0.0 1.8 Needle EMG: Fibs PW Fasc Pattern 0 0 0 2 Rate Polys Dur Amp 1+ Incr Incr Biceps brachii L Vastus lateralis L Pathology Muscle Biopsy : Right Deltoid • Features of Denervation: Atrophic angulated fibers Increased nuclear bags Increased CD56 Fiber Type 1 (slow) predominance No necrosis or infiltration Normal vasculature Increased intra-fascicular connective tissue No RRF, normal stainings (Oil Red O, PAS, SDH, cytochrome, Myophosphorylase, Acid phosphatase) In Summary… 53 yo. male with subacute progressive, symmetric, proximal weakness of arms and legs with associated unsteady gait, and hyporreflexia. Nerve conduction revealed a mixed sensory and motor neuropathy with both axonal degeneration and demyelination. Toxic Neuropathy Amiodarone Amiodarone • Di-iodinated benzofuran derivative initially developed in the 1960s as an anti-anginal agent. • Later used as a cardiac antiarrhythmic agent in the management of supraventricular and ventricular arrhythmias. • Approved for the treatment of refractory and lifethreatening ventricular arrhythmias. • Class III antiarrhythmic agent and shares the capacity to prolong the duration of action potentials and refractoriness in Purkinje fibers and ventricular muscle Adverse Effects • • Prevalence of side effects 15% in the first year, 50% long-term therapy An amphiphilic drug that forms intra-lysosomal lipid complexes in multiple tissues: Pulmonary Chronic Insterstitial Pneumonitis, ARDS, Organizing PNA, Amiodarone phosphoplipid complexes in air-spaces, decreased DLCO Thyroid Hyperthyroidism or Hypothyroidism Incidence affected by underlying thyroid status Skin Facial “blue man syndrome” Ocular Corneal microdeposits, optic neuropathy Cardiac Sinus bradycardia, Prolonged QT, potentiates Warfarin Neurologic Neurotoxicity • • • • • • • Tremor occurring in 43% and 39% in 2 large series 6 to 10 Hz action tremor, bilateral and symmetric clinically indistinguishable from essential tremor Ataxia 7% and 37% of cases in these same series associated with limb ataxia and other findings clearly suggesting cerebellar dysfunction Optic neuropathy insidious onset and slow progression of bilateral visual loss associated with optic disc swelling Myopathy without associated neuropathy, with a proximal pattern of weakness and occasional myalgia Basal ganglia dysfunction Encephalopathy Pseudotumor cerebri Neuropathy • Clinical Features : Symmetric, subacute to chronic, sensorimotor polyneuropathy Proximal or distal weakness Large-fiber sensory modalities are typically involved with or without small fiber dysfunction Occasionally, neuropathy is predominantly motor Not length-dependent Evolution may be rapid, mimicking Guillain-Barré Glove-stocking loss of all sensory modalities, depressed reflexes, and an ataxic gait • Electro-diagnostic and Lab Features: Demyelination and axonal involvement Reinnervation features “Myopathic” MUP’s rarely seen CK levels do not correlate CSF is normal Helps distinguish from GBS Histopathology Loss of large and small myelinated axons Muscle is less affected than nerve • Pathogenesis: Direct toxic effect on Schwann cells Cause of axon loss thoight to be “secondary.” Half-life (~53 days) predisposes to widespread drug accumulation Severity and likelihood of developing toxicity uncertain Age or dose not a risk factors Length of time receiving medication Medication metabolism? Dosing strategy of current era Literature Review Summary: Retrospective medical record analysis of cardiac patients treated with amiodarone at Mayo Clinic between 1998-2006. Neurologic effects that might be attributable to amiodarone were tabulated. Summary: Three patients developed peripheral neuropathy after taking amiodarone for more than 18 months. All had high serum concentrations of amiodarone and its desethyl metabolite; in one patient concentrations in a sural nerve biopsy were 80 times higher than in serum. Peripheral neuropathy is a complication of large doses of amiodarone taken over long periods. Summary: Two patients had slightly asymmetric, mixed, but primarily demyelinating sensorimotor polyneuropathy. One also had a substantial myopathy. The third had an acute neuropathy resembling GBS. Seemingly CK levels did not correlate with clinical or EMG evidence of mypathy. Histologic evaluations of peripheral nerves revealed demyelination, some axon loss and a variable number of characteristic lysosomal inclusions. After discontinuation of amiodarone, two patients improved and one died of cardiac arrhythmia. • Main Treatment : DISCONTINUING Amiodarone Reversible partially or almost completely in 16 months afterwards May lead to death secondary to cardiac problems Hospital Course • Amiodarone was discontinued by the 3rd day of hospitalization Neuropathy: Subjective improved strength and energy At discharge, able to ambulate 200 feet Follow up appointment LSU Neurology-Kenner Pleural effusion with interstitial disease, PFT: mild restrictive pattern with decreased DLCO Hypothyroidism Synthroid 50 mcg Atrial fibrillation and AICD Monitor ICD activations References • Alport A, Sander H. Clinical Approach to Peripheral Neuropathy: Anatomic Localization and Diagnostic Testing. Continuum 2012; 18:13-38. • Martinez-Arizala A, Sobol SM, McCarty GE, et al. Amiodarone neuropathy. Neurology 1983;33:643-645. Pulipaka U, Lacomis D, Omalu B. Amiodarone-Induced Neuromyopathy: Three cases and a review of literature. J Clin Neuromusc Disease 2002;3:97-105. Fraser Ag, McQueen IN, Watt AH, et al. Peripheral Neuropathy during long-term high dose amiodarone therapy. J Neurol Neursurg Psychiatry 1985; 48:576-578. Kang HM, Kang YS, Kim SH, Seong JK, Kang DY, Lee HY, Lee BS. Amiodarone induced hepatitis and polyneuropathy. Korean Journal of Internal Medicine 2007;22:225-229. Charness ME, Morady F, Scheinman MM. Frequent neurologic toxicity associated with amiodarone therapy. Neurology 1984;34:669-671. Orr C, Ahlskog, E. Frequency, Characteristics, and Risk Factors for Amiodarone Neurotoxicity. Arch Neurol 2009;66:865-869. • • • • •