* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download D.4 pH Regulation of the Stomach
Matrix-assisted laser desorption/ionization wikipedia , lookup
Genetic code wikipedia , lookup
Catalytic triad wikipedia , lookup
Peptide synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Butyric acid wikipedia , lookup
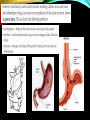
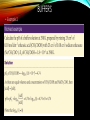
pH Regulation of the Stomach • The body keeps a tight control over the pH in cells and extra-cellular fluiids, as changes in the H+ concentration have significant effects on the activity of many molecules, especially enzymes. • The gastrointestinal tract generates and maintains different pH environments along its length, which play an important role in controlling the activity of digestive enzymes. The Helicobacter Pylori burrows into the mucus lining of the stomach , causing inflammation. This leads to a loss of mechanisms that protect the stomach wall from the acidic contents, and so tissue break down occurs. The treatment for chronic stomach inflammation and ulcers includes antiobiotic treatment and medines that reduce acid secretion BUFFERS BUFFERS A buffer is a solution composed of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers can resist drastic changes in pH when a small amount of strong acid or strong base is added. A buffer resists changes in pH because the acid species neutralizes OH- ions from a base and the basic species neutralizes the H+ ions from an acid. CALCULATING THE pH OF THE BUFFER • Consider an acid, HA, and one its salts, MA (M could be any cation from group 1). • The acid dissociation equilibrium is: BUFFERS BUFFERS Notice that if the acid and the salt have the same concentration, then pH = pKa. Also, if the base and the salt have the same concentration, then the pOH = pKb. BUFFERS • Example 1 What is the pH of a buffer that is 0.12 M in lactic acid, HC3H3O3, and 0.10 M in sodium lactate, NaC3H3O3. Ka for lactic acid is 1.4 x 10-4 pKa = -log 1.4 x 10-4 = 3.85 pH = 3.85 + log .10/.12 pH = 3.85 – 0.08 pH = 3.77 BUFFERS • Example 2, BUFFERS • Example 3, • Calculate the pH of a buffer composed of 0.12 M benzoic acid and 0.20 M sodium benzoate. Ka for benzoic acid = 6.3 x 10---5 pKa = - log Ka = -log 6.3 x 10---5 = 4.20 pH = 4.20 + log 0.20/0.12 pH = 4.20 + 0.22 pH = 4.42 PREPARATION OF A BUFFER • The simplest way to prepare a buffer is by choosing an acid with a pKa close to the desired pH. In this manner, equal amounts of acid and salt can be used. • Equal amounts of the acid and salt can be obtained by reacting the acid with enough strong alkali, such as NaOH, to change ½ of the acid into salt. • Example 1 Your text here PREPARATION OF A BUFFER • Another way a buffer can be prepared is by mixing a weak acid or weak base with a salt of that acid or base. • For example: 1. Adding sodium acetate to a solution of acetic acid or 2. Adding ammonium chloride to a solution of ammonia. PREPARATION OF A BUFFER • Example 2, How would you prepare 1 L of pH 5.00 buffer solution from 0.10 M butanoic acid solution and solid potassium butanoate? In this type of problem, assume the volume won’t change when the salt is added. 1. Obtain 1 L of butanoic acid. 2. Calculate the grams of potassium butanoate needed. 3. Mix the salt with the 1 L of acid. CHANGES IN pH OF A BUFFER To calculate the change in pH that occurs in a buffer solution when a strong acid or strong base is added: 1. Calculate the pH of the buffer before the addition of the strong acid or strong base. 2. Write the an equation for the reaction and determine how much of the salt and weak acid/base are present after the reaction (use ICE table). 3. Calculate the pH of the resulting solution. 4. Find the difference between the pH before and after the reaction. CHANGES IN pH OF A BUFFER CHANGES IN pH OF A BUFFER CHANGES IN pH OF A BUFFER If both the volume and the concentration of the solutions are given, then first find the number of moles. CHANGES IN pH OF A BUFFER CHANGES IN pH OF A BUFFER