* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download document
Immune system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
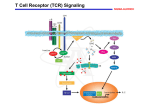
PHAR 423: Drugs in the Therapy of Transplant Rejection Dr. Thomas Abraham Spring 2004 Transplantation of organs Autograft: tissue from the same individual. Isograft: tissue from genetically identical donor e.g. monozygotic twins. Allograft: tissue from genetically distinct donor. Xenograft: tissue from donor of another species. Transplantation of organs • High demand for transplant tissue: about 64,000 recipients on waiting list in 1999 • Waiting period for kidney can be about 3 years! • Generally blood and skin tissues are rejected most rapidly following transplantation. • Organ transplant is usually followed by: 1. Hyperacute rejection – due to ABO blood group incompatibility. 2. Delayed-type hypersensitivity reaction – cell mediated immunity. Role of T Cells in Transplant Rejection • Cell-mediated immunity primarily carried out by CD4+ and CD8+ T cells. • Altered MHC molecules (type I and II) on graft tissue are recognized by T cells to initiate the immune reaction. • Alternatively antigen-presenting cells (dendritic cells, macrophages, passenger leukocytes) may process and present altered MHC molecules to T cells. • Stages of Rejection 1. Sensitization – macrophages engulf and process graft MHC molecules, proliferation of T cells after stimulation by APCs, release of cytokines (IL2, TNFb, IFNg). 2. Effector stage – infiltration of macrophages/neutrophils; CD8+-mediated killing of graft cells; IL-2 induced T cell proliferation & differentiation. Role of T Cells in Transplant Rejection • CD4+ T cells bind altered MHC molecules and become activated: begin to produce cytokines (mostly IL-2, TNFb, IFNg, etc.), cytokine receptors, and undergo proliferation and differentiation (T helper cell). • CD8+ T cells become activated by IL-2 (from CD4+ cells), recognize graft tissue and become cytotoxic T lymphocytes: bind MHC molecules of foreign cells and kill them directly. • T helper cells stimulate the activation of monocytes, which migrate to graft site and differentiate to macrophages: attack graft cells, cause inflammation, etc. Role of T Cells in Transplant Rejection Host Versus Graft Disease Histological Stain of biopsies from a transplanted Kidney 2 Weeks after transplant 2 Years after transplant Graft versus Host Disease Immune response against the host following bone marrow transplant Histological Stain of Liver tissue Skin condition after transplant Intracellular Signaling in T cells Intracellular Signaling in T cells • Binding of altered MHC molecule to TCR causes increase in intracellular calcium. Graft cell or APC MHC molecule • Calcium ion combines with calmodulin to bind and activate calcineurin (protein phosphatase 2B). T Cell Receptor T cell [Ca2+ ]i Calmodulin + Calcineurin (PP2B) P NFAT c NFAT c Pi Nucleus NFAT c NFAT n IL-2, IL-2 receptor • Calcineurin dephosphorylates cytoplasmic NFAT which then migrates into the nucleus of the T cell to increase the transcription of specific genes involved in T cell activation. Agents that modify Intracellular Signaling in T cells Cyclosporin A (Sandimmune®) • Isolated from fungus Tolypocladium inflatum; consists of 11 amino acid residues arranged in cyclic structure. • All amides are hydrogen-bonded or methylated. • Very lipophilic agent – administered in ethanol/castor oil mixture for IV solutions or as soft gelatin capsule or microemulsion for oral admin. • Cyclosporine binds to cyclophilin (intracellular receptor) to form a stable complex that inhibits the actions of calcineurin. Agents that modify Intracellular Signaling in T cells Cyclosporin (cont.) • Inactive calcineurin prevents T cell response (IL-2 production, differentiation, etc.), which blunts overall T cell-mediated immunity. • Cyclosporine also increases production of TGFb by various cells that inhibits the activation of T cells. • Oral bioavailability from soft gelatin capsule: slow and incomplete (20-50%); newer microemulsion formulation (Sandimmune Neoral®) has better oral bioavailability. • Peak plasma conc. occurs 1-4 hours after oral admin; delayed with fatty meals. Large volume of distribution with 50-60% of blood content in RBCs. High cyclophilin concentration allows heavy concentration in leukocytes. Agents that modify Intracellular Signaling in T cells Cyclosporin (cont.) • High hepatic metabolism to several products (mostly inactive); cyclic structure resistant to metabolism but extensive side-chain modification by CYP450 system. Predominant biliary excretion of metabolites (94%). Drug Interactions • Phenobarbital, phenytoin, rifampin etc. increase cyclosporine clearance; amphotericin B, erythromycin, ketoconazole decrease clearance. • Adverse effects: renal toxicity (in most pts.), hypertension (up to 50% of pts.), nervous system effects, hepatotoxicity, hirsutism, gingival hyperplasia and GI toxicity. • Used in kidney, pancreas, liver and cardiac transplants; also in severe rheumatoid arthritis and early type I diabetes. Agents that modify Intracellular Signaling in T cells Tacrolimus (Prograf®, FK506) • Isolated from streptomyces species; cyclic lactone structure. OH CH3O CH3 O H3C O OH CH2 O N O OH CH3 CH3 O CH3 O OCH3 OCH3 • Binds to different intracellular receptor immunophilin (FKBP-12) to form stable complex with calcineurin. Inhibition of calcineurin prevents T cell activation. • IV and oral preparations. Very high hepatic metabolism with predominant biliary excretion (99%). Toxicities include nephrotoxicity, CNS toxicities, hypertension, and metabolic toxicities. • Agents that interact with CYP3A4 e.g. metronidazole, diltiazem have resulted in elevated tacrolimus levels. Agents that modify Intracellular Signaling in T cells Sirolimus (Rapamune®, Rapamycin) • HO CH3 O • CH3 H H3C O N O OH H3C O HO O R O HO H3C O H3C CH3 • Isolated from Streptomyces species. Has comparative pharmacokinetics as tacrolimus with about 20% oral bioavailability and extensive hepatic metabolism and biliary excretion. • Does not inhibit calcineurin activity to prevent IL-2 gene expression. CH3 Inhibits T cell proliferation and differentiation, probably by inhibition of cellular protein kinases involved in regulating the cell division cycle (S6 kinase and cyclindependent kinases). May have actions to inhibit B cell function as well, at therapeutic doses. Agents that modify Intracellular Signaling in T cells Sirolimus (contd.) • Animal and human studies indicate sirolimus to be less nephrotoxic and hypertensive than cyclosporine. May increased serum cholesterol and triglycerides and potential to increase susceptibility to infections and lymphomas. • Interactions: dose adjustment when coadministered with diltiazem, cyclosporin, rifampin Glucocorticoid Agents Prednisone (Deltasone®), Prednisolone (Hydeltrasol®) CH2 OH O C CH3 OH CH3 O Prednisolone OH • Immunosuppressive effects by inhibiting T cell proliferation and prevention of cytokine gene expression. • May also decrease rejection by blocking inflammatory reactions (DTH) and infiltration of macrophages, neutrophils to site of graft. • Adverse effects: adrenal insufficiency, GI ulcers, hyperglycemia, osteoporosis and susceptibility to infection. • Used in acute transplant rejection, graft-vs-host disease, autoimmune disorders, allergic reactions, cytokine storm due to OKT3 Cytotoxic Agents Azathioprine (Imuran®) • N N O2N S N N CH3 H N N Purine antimetabolite that is converted to 6mercaptopurine in the presence of nucleophiles such as glutathione. • 6-mercaptopurine blocks de novo purine synthesis in lymphocytes to prevent T cell proliferation during antigen challenge. • Admin. oral and IV; good oral bioavailability of azathioprine with peak plasma levels at 1-2 h. Metabolites excreted in urine. Allopurinol prevents metabolism of 6-MP and leads to increased toxicity. • Adverse effects: affects mainly rapidly growing cells – bone marrow (leukopenia, thrombocytopenia); GI tract (NVD); hepatotoxicity. Cytotoxic Agents Mycophenolate Mofetil (CellCept®) O OH • CH3 O O O CH3 N O OCH3 2-morpholinoethyl ester of mycophenolic acid. Hydrolyzed to mycophenolic acid by hepatic/plasma esterases. Mofetil group improves oral bioavailability. • Mycophenolic acid potent noncompetitive, inhibitor of inosine monophosphate dehydrogenase (rate-limiting enzyme of de novo pathway) which is involved in the guanine nucleotide biosynthesis pathway. Leukocytes depend on this pathway for production of purines and thus more selective immunosuppression is achieved (little bone marrow toxicity). Inosine IMPD monophosphate • Xanthine monophosphate GTP RNA dGTP DNA Guanosine monophosphate Decreased leukocyte proliferation and decreased antibody production by B cells Cytotoxic Agents Mycophenolate (contd.) • Good oral absorption of mycophenolate mofetil (~94% bioavailability); rapidly converted to free acid which is further metabolized to glucuronide conjugates (inactive) that are renally excreted. High plasma protein binding of MPA (95%). • Adverse effects: diarrhea, GI dysfunction, neutropenia, viral infections. • Used to prevent renal or cardiac transplant rejection; often in combination with glucocorticoids and calcineurin inhibitors. • Other cytotoxic drugs used: cyclophosphamide, dactinomycin, mizoribine and leflunomide. Immunomodulating Antibodies Antithymocyte globulin (ATG) • Polyclonal antibodies from sheep, horse, rabbit or goat that have been immunized with thymic lymphocytes (immature). • The purified antibodies when administered IV bind to circulating T cells to cause leukopenia and decreased immune responsiveness (plasma half-life 3-9 days). • Adverse effects: serum sickness, nephritis, chills, fever, leukopenia, thrombocytopenia and skin rash. Antilymphocyte globulin (ALG, Thymoglubulin®) • Animals (usually rabbits) immunized with human lymphocytes for polyclonal antibodies or monoclonal antibodies are produced by recombinant technology. • Antibody binding to lymphocytes causes their opsonization and phagocytosis by macrophages, neutrophils; induction of complement cascade and cytotoxic cell killing. Immunomodulating Antibodies Muromonab-CD3 monoclonal antibody (OKT3, Orthoclone®) Mouse monoclonal antibody produced against human CD3 protein on T cells that is required for recognition of altered MHC of the graft along with TCR. Binding of muromonab-CD3 to CD3 results in lack of MHC binding to TCR (blunts immune response) and causes loss of circulating T cells. When T cells reappear in blood they are without TCR. Also causes release of cytokines by activating T cells which decreases subsequent immune response. Adverse effects: cytokine release syndrome, anaphylactoid syndrome, CNS toxicity and propensity to infection and neoplasias. • Used to decrease acute transplant rejection Immunomodulating Antibodies Daclizumab (Zenapax®), Basiliximab (Simulect®) Newer human/mouse monoclonal (recombinant) antibodies that bind to asubunit of IL-2 receptor on activated T cells. Behave as IL-2 receptor antagonists to minimize the stimulation, proliferation and differentiation of T cells. Since the antibodies are mostly of human origin with just the antigen-binding site from mouse, they are less immunogenic during repeated administration. Immunomodulators in Immune disorders Infliximab (Remicade®) • Chimeric anti-TNFa antibody neutralizes TNFa to prevent its actions • Used in rheumatoid arthritis, Crohn’s disease Etanercept (Embrel®) • Composed of the ligand binding domain of the TNFa receptor fused to the FC portion of IgG • Binds to and removes TNFa before it can bind to tissue receptors to produce disease.