* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 25.11.2011
Hygiene hypothesis wikipedia , lookup
Lymphopoiesis wikipedia , lookup
DNA vaccination wikipedia , lookup
Molecular mimicry wikipedia , lookup
Immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Innate immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
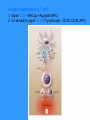
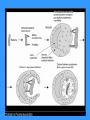
Antigen presentation
Antigen presentation
Antigen recognition
Antigen presentation to T cells
1. Signal TCR – MHC gp I+Ag peptid (APC)
2. Co-stimulatory signal CD 28 (T lymphocyte) – CD 80, CD 86 (APC)
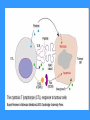
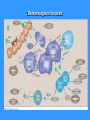
TH1 based
immune response
TH1 immune response
- inflammatory reaction
The role of TH1 cells is cooperation with macrophages
and their transformation in activated, which are capable
to produce NO through which destroy its intracellular
parasites
For the conversion to activated macrophages are
essential cytokines (IFNg) produced by TH1 cells
Activated macrophages secrete some cytokines (IL1, TNF, ...) that help to stimulate T cells and
stimulate local inflammation, which helps suppress
infection
Interaction between TH1 cells and macrophages is a
fundamental mechanism of delayed-type
immunopathological reactions (DTH Delayed-type
hypersensitivity)
The infected macrophage produces protein
fragments derived from intracellular parasites, some
of which are presented on the surface by MHC gp
class II
Macrophages and dendritic cells stimulated by certain
microorganisms produce IL-12
TH precursor, which detects the infected macrophage and
receives signals through the TCR, CD 28 and receptor for
IL-12 and other adhesion and signaling molecules
proliferates and differentiates to the effector TH1 cells that
produce IFNg and IL-2.
IFNg promotes transformation of macrofages in activated
IL-2 is an autocrine growth factor for TH1 cells
Interaction between APC and TH precursor
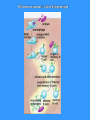
TH2 based
immune response
TH2 immune response – help to B-lymphocytes
The basic function of TH2 cells is the cooperation with B
lymphocytes (which were stimulated by Ag) by cytokines
(IL-4, IL-5, IL-6) and direct intercellular contact
For stimulation of B lymphocytes is usually necessary
cooperation between APC → TH2 cell → B lymphocyte
In the case of the minimal model, where the B cell
becomes a good APC (CD80, CD86) is sufficient
cooperation between TH2 cell → B lymphocyte
TH precursor, which detects the infected macrophage
and receives signals through the TCR, CD 28 receptor for
IL-4 receptor and IL-2 and other adhesion and signaling
molecules proliferates and differentiates in the effector
TH2, which provide B lymphocytes auxiliary signals via
cytokines secreted by IL-4, IL-5, IL-6 and adhesion
molecules through CD 40L, which bind to the
costimulatory receptor on B lymphocytes CD 40
Interaction between CD40 (B lymphocytes) and CD40L
(TH2 cells) is essential for the initiation of somatic
mutations, izotype switching and formation of memory
cells
IL-4, IL-5, IL-6: stimulation of B lymphocytes
TH2 immune response – help to B-lymphocytes
Assistance to B lymphocytes
Specific direct help to B lymphocytes:
TH2 lymphocytes assisting B lymphocytes that were
stimulated by the same Ag, which caused the rise of TH2
To stimulate the secretion of cytokines by TH2 cell is
sufficient signal via the TCR (signal through a
costimulatory receptor CD28 is no longer necessary)
One clone of TH2 cells can provide specific assistance to
B lymphocytes of different specificities (must present the
relevant Ag peptides by MHC gp II, which are recognized
by TCR)
Assistance to B lymphocytes
Indirect help to B cells ("bystander help"):
TH2 lymphocytes assisting B lymphocytes that were
stimulated by another Ag than that which caused the
rise of TH2
Contact between TH2 cell → B lymphocytes via adhesion
molecules, cytokine secretion, binding CD40-CD40L
Danger of activation autoreactive B lymphocytes
Mutual regulation of activities TH1versus TH2
Whether the TH precursor cell will develop into TH1 or
TH2 decides cytokine ratio of IL-12 and IL-4
IL-12 is produced by macrophages and dendritic cells
stimulated by certain microorganisms
IL-4 is produced by activated basophils and mast cells
TH1 cytokines (mainly IFNg) inhibit the development
of TH2 and stimulate the development of TH1 (IL-2
stimulates also TH2)
Cytokines produced by TH2 (IL-4, IL-10) inhibit the
development of TH1 and stimulate the development of
TH2
TC based
immune response
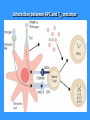
Cytotoxic T lymphocytes stimulation
TC recognize cells infected with viruses or other
intracellular parasites, and some tumor cells
Precursor of TC, which recognizes a complex
of MHC gp I- antigenic peptide on the surface of APC via
TCR and receives signals via CD 28 proliferates and
differentiates to clone mature effector cytotoxic cells
(CTL); TH1 cells help to TC by production IL-2
Effector TC are spread by bloodstream into tissues; for
activation of cytotoxic mechanisms is sufficient signal
through the TCR (signal through a costimulatory
receptor CD28 is no longer necessary)
Tc effector functions
Cytotoxic granules containing perforin and granzymes
(perforin creates pores in the cytoplasmic membrane of
target cell, in some cases may lead to osmotic lysis of
the target cell, formed pores in the cell receiving
granzymes that cause the target cell to die by
apoptosis.
Fas ligand (FasL) - which binds to the apoptotic
receptor Fas (CD95) presented on the surface of many
different cells (also on the surface of TC)
TNFb
Antiinfection immunity
Jitka Ochotná
Defense against extracellular bacteria
gram-negative, gram-positive cocci, bacilli
for their elimination is necessary opsonization (C3b, lectins,
antibodies ...)
neutrophilic granulocytes are chemotactic attracting to the site
of the infection (C5a, C3a and chemotactic products of bacteria)
absorbed bacteria are destroyed by the microbicidal systems
(products NADP-H oxidase, hydrolytic enzymes and bactericidal
substances in lysosomes)
phagocytes production of proinflammatory cytokines (IL-1, IL-6,
TNF) that induce an increase in temperature, metabolic response
of the organism and synthesis of acute phase proteins
in later stages of infection are stimulated antigen-specific
mechanisms
plasma cells initially produce IgM isotype after isotype switching
produce
IgG1 and IgA (opsonization)
sIgA protect against intestinal and respiratory infections
caused by bacteria
bacteria with a polysaccharide capsule may cause T-independent
IgM antibody production (after the establishment to the bacteria
activate the classical complement path)
after infection persist IgG, IgA (protective effect)
and memory T and B lymphocytes
in
the defense against bacterial toxins apply neutralizing
antibodies (Clostridium tetani and botulinum ...)
bacterial Lipopolysaccharide (LPS) stimulates big
number of monocytes to release TNF, which can cause
septic shock
extracellular bacterial infections are especially at risk
individuals with disorders in the function of phagocytes,
complement and antibody production
Defense against intracellular bacteria and molds
mycobacteria, some yeasts and molds
intracellular parasitism is given
by the ability of microorganisms to
escape microbicidal mechanisms of phagocytes
macrophages, which absorbed them, produce IL-12 → TH1
differentiation, production of IFNg and membrane TNF →
activation of macrophages and induction of iNOS
plasma cells under the influence of IFNg produce IgG2,
immune complexes containing IgG2 bind to Fc receptors
on macrophages and thus stimulate
in the defense against intracelular parasites, which
escape from phagolysosomes apply TC lymphocytes
intracellular microorganisms infections are at risk
individuals with certain disorders of phagocytes and
defects of T lymphocytes
Defense against viruses
interferons
- in infected cells is induced production of IFNa and
IFNb (prevents viral replication and in uninfected cells cause the
anti-virus status); IFNg stimulates the conversion to activated
macrophages (iNOS)
NK cells - ADCC (Antibody-dependent cell-mediated cytotoxicity)
= cytotoxic reaction depends on the antibodies; the NKlymphocyte recognizes cell opsonized with IgG by stimulation Fc
receptor CD16 and then activate cytotoxic mechanisms
(degranulation)
infected macrophages produce IL-12 (a strong activator of NK
cells)
Defense against viruses
in the defense against cytopathic viruses mostly applied
antibodies:
sIgA inhibit mucosal adhesion of viruses
(defense against respiratory viruses and
enteroviruses)
neutralizing IgG and IgM antibodies activate the
classical pathway of complement
IgA and IgG derived in viral infection have a
preventive effect in secondary infection
effector
TC lymphocytes destroy infected cells in direct contact
(granzym/perforin; FasL) and by produced cytokines
lymfotoxin)
some viruses after infection integrate into the host genome,
where persist for years (varicella zoster, EBV, papillomavirus)
by these infections are at risk individuals with T lymphocyte
immunodeficiency and with combined immune disorders
increased susceptibility to herpes infections in individuals with
dysfunction of NK cells
Defense against protozoa parasites
Toxoplasma gondii, Leishmania,
Trypanosoma
defense against protozoa parasites is similar to bacteria
extracellular parasites - antibodies
intracellular parasites - TH1 lymphocytes and activated
macrophages
Defense against multicelular
parasites
mast cells, basophils and eosinophils
TH2 stimulation under the influence of IL-4 (mast cells and
other APC stimulated by parasite)
TH2 stimulate B cells with BCR-specific parasite antigens
isotype switching under the influence of IL-4 in IgE
IgE bind to FceRI on mast cells and basophils (antigenspecific receptors)
establish of multivalent antigen (multicellular parasite) using
the IgE to highafinity Fc receptor for IgE (FceRI)
aggregation of several molecules FceRI
initiate mast cell degranulation (cytoplasmic granules mergers
with the surface membrane and release their contents) histamine
activation of arachidonic acid metabolism (leukotriene C4,
prostaglandin PGD2) - amplification of inflammatory
responses
production of cytokines (TNF, TGFb, IL-4, 5,6 ...)
in later stages are activated TH1 and are produced
antibodies of other classes
eosinophils fagocyte complexes of parasitic particles
with IgE via their receptors for IgE
eosinophils use against parasites extracellular
bactericidal substances released from granules
(eosinophil cationic protein, protease)
Possibilities of the therapeutic
effect on the immune system
Causal treatment
a) stem cell transplantation
for serious congenital disorders of the immune system (some
lymphoproliferative and myeloproliferative disorders)
complications: infectious complications
Graft-versus-host
obtaining stem cells - the collection from shovel hip bone
- from umbilical cord blood
- from peripheral blood after stimulation
with GM-CSF
b) gene therapy
with a suitable expression vector is introduced functional
gene (to replace dysfunctional gen) into the lymphocytes
or stem cells
used as a treatment for some cases of SCID
Substitution treatment
autologous stem cell transplantation following
chemotherapy and radiotherapy
treatment with intravenous immunoglobulin (derived
from plasma of blood donors)
substitution of C1 inhibitor for hereditary angioedema
substitution of erythropoietin in patients with chronic
renal failure
substitution of G-CSF in agranulocytosis
Non-specific immunomodulation therapy
Immunomodulation = medical procedure to adjust the disrupted
immune function
a) non-specific immunosuppressive therapy
nonspecific = affects not only autoreactive and aloreactive
lymphocytes, but also other components of
immunity (risk of reduction antiinfectious
and anti-tumor immunity)
- used for treatment of autoimmune diseases, severe allergic
conditions and for organ transplantation
corticosteroids - anti-inflammatory, immunosuppressive effects
- blocking the activity of transcription factors (AP-1,
NFkB)
- suppress the expression of genes (IL-2, IL-1,
phospholipase A, MHCgpII, adhesion molecules)
- inhibition of histamine release from basophil
- higher concentrations induce apoptosis
- prednison, methylprednison
immunosuppressants affecting the metabolism of DNA
- cyclophosphamide (alkylating agent)
- methotrexate (antimetabolite)
- azathioprine (purine analogue)
immunosuppressant selectively inhibiting T lymphocyte
- cyclosporin A (inhibits the expression of IL-2
and IL-2R in activated T lymphocytes)
- tacrolimus, rapamycin
- monoclonal antibody anti-CD3
(immunosuppression after transplantation,
treatment of rejection crises)
Immunoglobulins in the immunosupressive treatment
b) anti-inflammatory and antiallergic treatment
nonsteroidal anti-inflammatory drugs
antihistamines - blocking H1 receptor
- reduce the expression of adhesion molecules
- reduce the secretion of histamine ...
inhibitors of inflammatory cytokine
- receptor antagonist for IL-1
- monoclonal antibodies against TNF
- thalidomide (TNF inhibitor)
c) non-specific immunostimulant therapy
imunostimulancia - stimulate the immune system
synthetic immunomodulators
- Methisoprinol (Isoprinosine) - used in viral
infections with more severe or relapsing course
bacterial extracts and lysates
- Broncho-Vaxom – prevention of recurrent
respiratory tract infections
- Ribomunyl
products of the immune system
- IL-2 - renal adenocarcinoma
- IFNa, IFNb - viral hepatitis, some leukemia
- Erythropoietin
- G-CSF, GM-CSF - neutropenia
thymic hormones
transfer factor
Antigen-specific
immunomodulatory therapy
specific immunomodulation = induce immune
response or tolerance to specific antigen
a) Active immunization
b) Passive immunization
c) specific immunosuppression
a) active immunization
= use of antigen to induce an immune response that can
later protect against a pathogen bearing the antigen (or
antigen like him)
immunization vaccines made from inactivated or
attenuated microorganisms or their antigens
(polysaccharide capsule, toxins)
creates long-term immunity
activate cellular and antibody immunity
administration of antigen injectable, oral
prophylaxis
risk of causing infection or anaphylactic reactions
b) passive immunization
natural - transfer of maternal antibodies in fetal blood
therapeutically - the use of animal antibodies against various
toxins (snake toxins, tetanus toxin,botulinum
toxin)
prophylaxis - the human immunoglobulin from immunized
individuals (hepatitis A, rabies, tetanus)
- Anti-RhD antibodies - preventing maternal
immunization with RhD+ fetus
provides a temporary (3 weeks) specific humoral immunity
the risk of inducing anaphylactic reactions
c) specific immunosuppression
= induction of tolerance against a specific antigen
the clinical studies:
induction of tolerance by oral administration of antigen
allergen immunotherapy (pollen, insect poisons)
THANK YOU FOR ATTENTION