* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antibodies, structure. Classes of Immunoglobulines
Psychoneuroimmunology wikipedia , lookup
Immune system wikipedia , lookup
Complement system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Major histocompatibility complex wikipedia , lookup
Innate immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Molecular mimicry wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Monoclonal antibody wikipedia , lookup
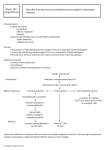
Lector Tvorko M. S. ANTIBODIES (IMMUNOGLOBULINS) Antibodies are globulin proteins (immunoglobulins) that react specifically with the antigen that stimulated their production. They make up about 20% of the protein in blood plasma.. There are five classes of antibodies: IgG, IgM, IgA, IgD, and IgE. IMMUNOGLOBULIN STRUCTURE Immunoglobulins are glycoproteins made up of light (L) and heavy (H) polypeptide chains. The terms "light" and heavy" refer to molecular weight; The simplest antibody molecule has a Y shape and consists of four polypeptide chains: two H chains and two L chains. The four chains are linked by disulfide bonds. An individual antibody molecule always consists of identical H chains and identical L chains. The Immune system includes 5 classes of Immunoglobulin (Ig) Heavy (H) polypeptide chains Class of immunoglobulin Ig G Ig M Ig A Ig E Ig D Ig G - 22,,22 Ig M - (22)5, (22)5 Ig A - (22)n , (22)n,, Ig E - 22, 22 IgD - 22, 22 If an antibody molecule is treated with a proteolytic enzyme such as papain, peptide bonds in the "hinge" region are broken, producing two identical Fab fragments, which carry the antigen-binding sites, and one Fc fragment, which is involved in placenta! transfer, complement fixation, attachment site for various cells, and other biologic activities IMMUNOGLOBULIN CLASSES IgG. Each IgG molecule consists of two L chains and two H chains linked by disulfide bonds (molecular formula H2L2). Because it has two identical antigen-binding sites, it is said to be divalent. IgG is the predominant antibody in the secondary-response and constitutes an important defense against bacteria and viruses. IgG is the only antibody to cross the placenta only its Fc portion binds to receptors on the surface of placental cells. It is therefore the most abundant immunoglobulin in newborn. IgG is one of the two immunoglobulins that can activate complement and opsonizes. IgM is the main immunoglobulin produced early in the primary response. It is present as a monomer on the surface of virtually all B cells, where it functions as an antigen-binding receptor In serum, it is a pentamer composed of 5 H2L2 units plus one molecule of J (joining) chain. Because the pentamer has 10 antigen-binding sites, it is the most efficient immunoglobulin in agglutination, complement fixation (activation), and other antibody reactions and is important in defense against bacteria and viruses. It can be produced by the fetus in certain infections. It has the highest avidity of the immunoglobulins; its interaction with antigen can involve all 10 of its binding sites. IgA is the main immunoglobulin in secretions such as colostrum, saliva, tears, and respiratory, intestinal, and genital tract secretions. It prevents attachment of bacteria and viruses to mucous membranes. Each secretory IgA molecule consists of two H2L2 units plus one molecule each of J (joining) chain and secretory component. The secretory component is a polypeptide synthesized by epithelial cells that provides for IgA passage to the mucosal surface. It also prelects IgA from being degraded in the intestinal tract. In serum, some IgA exists as monomericH2L2. IgE is medically important for two reasons: (1) it mediates immediate (anaphylactic) hypersensitivity, and (2) it participates in host defenses against certain parasites, eg, helminths (worms). The Fc region of IgE binds to the surface of mast cells and basophils. Although IgE is present in trace amounts in normal serum (approximately 0.004%), persons with allergic reactivity have greatly increased amounts, and IgE may appear in external secretions. IgE does not fix complement and does not cross the placenta. IgD. This immunoglobulin has no known antibody function but may function as an antigen receptor; it is present on the surface of many B lymphocytes. It is present in small amounts in serum. Major Functions of Human Immunoglobulins • Function IgM Main Ig during Primary Response (Early antibody). Fixes Complement (most effectively). • IgG Main Ig during Secondary Response (late antibody). Opsonization. Fixes Complement. Neutralizes Toxins, Viruses. • IgA Secretory mucosal Ig Prevents invasion from gut mucosa. • IgE Immediate Hypersensitivity. Mast cell and Basophil reactions. Activates Eosinophils in helminth infection • IgD Function Unknown. Mostly on the Surface of B cells. Immunoglobulins • Antibody-mediated mechanisms of antigen disposal Binding of antibodies to antigens inactivates antigens by Viral neutralization (blocks binding to host) and opsonization (increases phagocytosis) Agglutination of antigen-bearing particles, such as microbes Precipitation of soluble antigens Complement proteins Bacteria Virus Activation of complement system and pore formation MAC Pore Soluble antigens Bacterium Enhances Phagocytosis Macrophage Foreign cell Leads to Cell lysis • Class II MHC molecules, located mainly on dendritic cells, macrophages, and B cells – Display antigens to helper T cells Antigenpresenting cell Microbe 1 A fragment of foreign protein (antigen) inside the cell associates with an MHC molecule and is transported to the cell surface. Antigen fragment 1 Class II MHC molecule 2 2 The combination of MHC molecule and antigen is recognized by a T cell, alerting it to the infection. (b) T cell receptor Helper T cell • Class I MHC molecules, found on almost all nucleated cells of the body – Display peptide antigens to cytotoxic T cells Infected cell Antigen fragment 1 1 A fragment of foreign protein (antigen) inside the cell associates with an MHC molecule and is transported to the cell surface. Class I MHC molecule T cell receptor 2 (a) Cytotoxic T cell 2 The combination of MHC molecule and antigen is recognized by a T cell, alerting it to the infection. • The role of helper T cells in acquired immunity 1 After a dendritic cell engulfs and degrades a bacterium, it displays bacterial antigen fragments (peptides) complexed with a class II MHC molecule on the cell surface. A specific helper T cell binds to the displayed complex via its TCR with the aid of CD4. This interaction promotes secretion of cytokines by the dendritic cell. Cytotoxic T cell Dendritic cell Bacterium Peptide antigen Class II MHC molecule Helper T cell Cell-mediated immunity (attack on infected cells) TCR 2 3 1 CD4 Dendritic cell Cytokines 2 Proliferation of the T cell, stimulated by cytokines from both the dendritic cell and the T cell itself, gives rise to a clone of activated helper T cells (not shown), all with receptors for the same MHC–antigen complex. B cell 3 The cells in this clone secrete other cytokines that help activate B cells and cytotoxic T cells. Humoral immunity (secretion of antibodies by plasma cells) 1 After a macrophage engulfs and degrades 2 a bacterium, it displays a peptide antigen complexed with a class II MHC molecule. A helper T cell that recognizes the displayed complex is activated with the aid of cytokines secreted from the macrophage, forming a clone of activated helper T cells (not shown). A B cell that has taken up and degraded the same bacterium displays class II MHC–peptide antigen complexes. An activated helper T cell bearing receptors specific for the displayed antigen binds to the B cell. This interaction, with the aid of cytokines from the T cell, activates the B cell. 3 The activated B cell proliferates and differentiates into memory B cells and antibody-secreting plasma cells. The secreted antibodies are specific for the same bacterial antigen that initiated the response. Bacterium Macrophage Peptide antigen Class II MHC molecule B cell 2 1 TCR 3 Clone of plasma cells Endoplasmic reticulum of plasma cell CD4 Cytokines Helper T cell Secreted antibody molecules Activated helper T cell Clone of memory B cells Humoral Immune response • The activated cytotoxic T cell – Secretes proteins that destroy the infected target cell 1 A specific cytotoxic T cell binds to a class I MHC–antigen complex on a target cell via its TCR with the aid of CD8. This interaction, along with cytokines from helper T cells, leads to the activation of the cytotoxic cell. 2 The activated T cell releases perforin molecules, which form pores in the target cell membrane, and proteolytic enzymes (granzymes), which enter the target cell by endocytosis. Cytotoxic T cell 3 The granzymes initiate apoptosis within the target cells, leading to fragmentation of the nucleus, release of small apoptotic bodies, and eventual cell death. The released cytotoxic T cell can attack other target cells. Released cytotoxic T cell Perforin Cancer cell Granzymes 1 TCR Class I MHC molecule Target cell 3 CD8 2 Peptide antigen Apoptotic target cell Pore Cytotoxic T cell Immune synapsis