* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 7th seminar 2013 Complement system
Drosophila melanogaster wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Biochemical cascade wikipedia , lookup
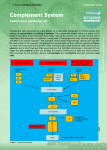
THE COMPLEMENT SYSTEM Sections from chapter 2 and 11 in Parham’s book Complement system The complement system is a set of about 30 soluble proteins, constitutively produced by the liver, that are found in the blood, lymph and extracellular fluids, and act against extracellular pathogens. Complement activation proceeds by a cascade of enzymatic reactions (proteases), in which each protease cleaves and activates the next enzyme in the pathway. THE CENTRAL COMPONENT OF THE COMPLEMENT SYSTEM C3 CGEQ CLEAVAGE SITE One of the proteins present at the highest concentration in serum 1.2mg/ml (3 900 000 000 000 000 molecules/ml) C3 CGEQ CGEQ OH OH R RR O Bacterium CGEQ C3a C3b Inflammation Binding CGEQ OH R OH R O R ROH Cell THE CENTRAL COMPONENT OF THE COMPLEMENT SYSTEM Complement fixationCovalent binding of C3b to the pathogens’ surface The alternative pathway The alternative soluble C3 convertase The alternative C3 convertase C3bBb AMPLIFICATION OF THE COMPLEMENT CASCADE inactive precursors limited proteolysis enzyme activating surface Regulation of the complement system Positive regulation Negative regulation (Inhibits both alternative and classical convertases) DAF and MCP Negative regulatory proteins on human cells protecting them from complement-mediated attack MCP binds to sialic acid on the surface of human cells and prevents the complement activation GLYCOSYLATION OF PROTEINS IS DIFFERENT IN VARIOUS SPECIES Prokariotic cells Eukariotic cells Sialic acid Glucoseamine Mannose Galactose Neuraminic acid (Sialic acid) The classical pathway THE C1 COMPLEX C1qR binding by Phagocytes Cleavage of C4 and C2 components Collagen „legs” Gobular „heads” Binding the Fc part of an antibody Immunoglobulin Fragments: Structure/Function Relationships antigen binding complement binding site binding to Fc receptors C1 component ‘heads’ placental transfer Association between native and adaptive immunity Only the antigen-linked antibodies are able to associate to complement. Low affinity binding to the C-terminal of an antibody Multiple interactions with immune complexes The classical C3 convertase C4bC2a CRP binds to phosphocholine component of the lipopolisaccharides in bacterial and fungal cell wall but not to phosphocholine component of phospholipids on human cell membranes! ACUTE-PHASE RESPONSE INCREASES THE SUPPLY OF INNATE IMMUNITY MOLECULES C-reactive protein CRP levels can increase up to 1000-fold during an acute-phase response! One of the major function of C1 INHIBITOR C1q binds to IgM on bacterial surface C1q binds to at least two IgG molecules on bacterial surface Binding of C1q to Ig activates C1r, which cleaves and activates the serine protease C1s C1INH dissociates C1r and C1s from the active C1 complex The Mannose-binding Lectin pathway • Binds Mannose-containing carbohydrates of bacteria, fungi, protozoans and viruses • Similar to C1q protein in triggering a complement cascade • MASP-1 and 2 have common gene ancestors with C1r and C1s • A member of the Collectin family GLYCOSYLATION OF PROTEINS IS DIFFERENT IN VARIOUS SPECIES Prokariotic cells Eukariotic cells Mannose Glucoseamine Mannose Galactose Neuraminic acid (sialic acid) ACUTE-PHASE RESPONSE INCREASES THE SUPPLY OF INNATE IMMUNITY MOLECULES Mannose-binding lectin MBL levels can increase up to 1000-fold during an acute-phase response! * SP-A and SP-D belong to the collectin family as well, opsonyzing pathogens in the lung Local inflammatory responses can be induced by the small complement fragments C3a, C4a, and especially C5a Opsonization C3b Bacterium complement receptors Ex:CR1, CR3, CR4 macrophage Complement receptors Name Ligand Expression CR1 C3b>C4b, iC3b RBC, Mo/MØ, Gr, B Act-T, FDC C3d, C3dg, iC3b EBV, IFNa, CD23 B, activated T, FDC iC3b> C3dg, C3d ICAM-1, LPS, fibrinogen Mo/MØ, Gr, NK Mo/MØ, Gr, NK CD11c/CD18 iC3b, C3dg, C3d Fibriogen C3aR C3a M, B, Gr, Mo/MØ, Trombocites, simaizom, Neur C5aR C5a,, des-Arg-C5a M, B, Mo/MØ, Trombocytes, SMC, Neur C1qR C1q collagen part B, NGr, Mo/MØ, endothel C1qRp C1q Phagocytes CD35 CR2 CD21, CD21L CR3 CD11b/CD18 CR4 Membrane attack complex (MAC) C3bBbC3b = alternative C5 convertase Or C4bC2aC3 = classical C5 convertase The membrane-attack complex assembles to generate a pore in the lipid bilayer membrane MAC in the cell membrane CD59 prevents assembly of terminal complement components into a membrane pore Diseases caused by deficiencies in the complement pathways Complement protein Effects of deficiency C1, C2, C4 Immune-complex diseases C3 Susceptibility to a wide range of pyogenic infections C5-C9 Susceptibility to Neisseria Factor D, Properdin Susceptibility to capsulated bacteria and Neisseria but no Immune-complex disease Factor I Similar to C3 deficiency DAF, CD59 Autoimmune-like conditions including paroxysmal nocturnal hemoglobinuria (PNH) C1INH Hereditery angioneurotic edema (HANE) Immune complex diseases Early components of the classical pathway (C1-C4) are necessary for the elimination of immune complexes! Attachment of the complement components to the soluble immune complexes allows them to be transported, or ingested and degraded by CR-bearing cells. Deficiencies in these components lead to the accumulation of immune complexes in the blood, lymph and extracellular fluid and their deposition in tissues. Damage is caused by the deposition itself and by the activation of phagocytes causing inflammation. These may include: Pyogenic infections Systemic Lupus Erythematosus Vasculitis Glomerulonephritis Paroxysmal Nocturnal Hemoglobinuria (PNH) Acqired clonal mutation of PIG-A gene no GPI enchor proteins on RBCs No expression of the complement regulatory proteins CD59 and DAF on these RBCs episodes of complement-mediated RBCs lysis hemolytic anemia Symptoms include: Anemia (tiredness, shortness of breath, palpitations) Hemoglobin in the urine 40% develope thrombosis Therapy include: Anti-C5-Mab, transfusion, immunosuppression and BM transplantation. Hereditary Angioneuretic Adema (HANE) Deficiency in C1INH complement regulatory protein. The C1INH is a serine protease inhibitor that regulates the C1 complex and complement activation as well as inhibiting proteins in the coagulation cascade. Symptoms include: swellings of skin, gut and respiratory tracts serious acute abdomenal pain, vomiting Therapy include: C1INH from donor blood, Androgens and other bradykinin inactivators Supplementary materials Major regulating factors of complement system C1Inh: C1-inhibitor (serine-protease inhibitor, can affect in many steps) Factor H: inhibits C3-convertase of alternative pathway, co-factor of factor I, cleaves C4b and C3b Properdin: stabilizes convertases of alternative pathway DAF: Decay Accelerating Factor MCP: Membrane Cofactor Protein CD59: inhibits the linking of C9 and C8 Regulation of complement system Factor I a-2macrogl C1Inh DAF C4bp CR1 MCP LECTIN PATHWAY HRF C-pept.ase N CD59 Properdin S-protein DAF positive feedback Fact-H CR1 MCP Factor I membrane protein soluble molecule Complement receptors Name Ligand Expression CR1 C3b>C4b, iC3b RBC, Mo/MØ, Gr, B Act-T, FDC C3d, C3dg, iC3b EBV, IFNa, CD23 B, activated T, FDC iC3b> C3dg, C3d ICAM-1, LPS, fibrinogen Mo/MØ, Gr, NK Mo/MØ, Gr, NK CD11c/CD18 iC3b, C3dg, C3d Fibriogen C3aR C3a M, B, Gr, Mo/MØ, Trombocites, simaizom, Neur C5aR C5a,, des-Arg-C5a M, B, Mo/MØ, Trombocytes, SMC, Neur C1qR C1q collagen part B, NGr, Mo/MØ, endothel C1qRp C1q Phagocytes CD35 CR2 CD21, CD21L CR3 CD11b/CD18 CR4 COMPLEMENT SYSTEM CLASSICAL PATHWAY Antigen-antibody complex C1q, C1r, C1s Serin protease C4, C2 C4a* MB-LECTIN PATHWAY ALTERNATIVE PATHWAY Mannose Pathogen surface MBL MASP-1/MASP-2 C3 B, D Serin protease C4, C2 C3 CONVERTASE C3a, C5a C3b Inflammatory peptid mediators Phagocyte recruitment Opsonization Binding to phagocyte CR Immune complex removal Terminal C5b – C9 MAC Pathogen/cell lysis Deficiencies of complement system – cascade molecules Deficiencies of complement system – regulatory molecules, receptors Hereditary angioneurotic edema (HANE) (hereditary C1INH defect) • 17-year old boy - severe abdominal pain (frequent sharp spasms, vomiting) • appendectomia normal appendix • similar symptoms occured repeatedly earlier in his life with watery diarrhea • family history of prior illness • immunologist’s suspicion: hereditary angioneurotic edema • level of C1INH: 16% of the normal mean • daily doses of Winstrol (stanozolol) – marked diminution in the frequency and severity of symptoms • purified C1INH intravenously became avaible by the time Main symptoms: • swellings of skin, guts, respiratory tracts • serious acute abdomenal pain, vomiting • larynx swelling – may cause death Treatment: • iv C1INH • kallikrein and bradykinin receptor antagonists Child with symptomes of HANE Pathogenesis of hereditary angioneurotic edema activation of XII factor Inhibition by C1INH in many steps • bradykinin and C2-kinin: enhance the permeability of postcapillar venules activation of kallikrein activation of proactivator by contraction of endothel • holes in the venule walls • edema formation cleveage of kininogen to generate bradykinin, vasoactive peptide • C1 is always active without cleveage of C2a to generate C2-kinin, vasoactive peptide cleveage of plasminogen to generate plasmin cleveage of C2 to generate C2a activation of C1 activating surface because plasmine is always active Questions hereditary angioneurotic edema 1. Activation of complement system results in the release of histamine and chemokines, which normally produce pain, heat and itching. Why is the edema fluid in HANE free of cellular components, and why does the swelling not itch? - In HANE, C4b and C2b both generated free in plasma because plasmine always actives the C1 - There are not an activating surface, so C4b are not able to bind to a surface, so it is rapidly inactivated. The concentration of C4b and C2b are relatively low, no C3/C5 convertase is formed. C3 and C5 are not cleaved and C3a and C5a are not generated. After the complement activation histamine do not release which is caused by C3a Without C5a there is no cell recruitment BUT there are C2a-kinin and bradykinin which cause edema. 2. Which complement component levels will be decreased? Why? C2 and C4, because of the continous cleavage by activated C1. Questions hereditary angioneurotic edema 3. Would you expect the alternative pathway components to be low, normal or elevated? C1 plays no part in the alternative pathway. This pathway is not affected. 4. What about the levels of the terminal components? The unregulated activation of the early components does not lead to the formation of the C3/C5 convertase, so the terminal components are not abnormally activated. 5. Despite the complement deficiency in patients with HANE, they are not unduly susceptible to infection. Why not? The alternative pathway of complement activation is intact and these are compensated for by the potent amplification step from the alternative pathway. 6. How might you decide the background of the laryngeal edema (HANO or anaphylactic reaction)? If the laryngeal edema is anaphylactic, it will respond to epinephrine. If it is due to HANO, it will not, C1INH needed. Paroxysmal nocturnal hemoglobinuria (PNH) • Acqired clonal mutation of PIG-A gene – no GPI-enchored proteins in the the cell membrane • CD59 (upper pic) and CD55 complement regulatory proteins • No CD59 and/or CD55: PNH patients are highly susceptible to complement-mediated lysis (lower pics). • Eleveted levels of TF derived from complement-damaged leukocytes Paroxysmal nocturnal hemoglobinuria (PNH) symptoms and therapy • Haemolytic anaemia and associated symptoms • Specific th.: eculizumab (Soliris - anti-C5 monoclonal antibody) • Haemoglobin and its products in the urine • Curative th.: bone marrow transplantation • Thrombosis: in brain veins, mesentheric veins, vv. hepaticae (Budd-Chiarisyndrome) • Alternative th.: steroids (general immunosuppression) • transformation to acut myelogenous leukemia (AML), aplastic anaemia, myelodisplastic syndrome (MDS) • Anticoagulants: sc. heparin p.o. kumarin • Iron replacement • Transfusion (filtered-irradiated blood)