* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Virus-induced immunosuppression
Lymphopoiesis wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
DNA vaccination wikipedia , lookup
Immune system wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Henipavirus wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Hepatitis B wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
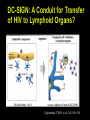
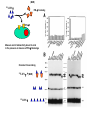
Virus-induced immunosuppression Infections usually stimulate immune response, but some infections can cause global immune suppression or specifically dampen one arm of immune system Mechanism Virus Host Degree of Immunosuppression Virusspecific or broad Attack on one or more ce lls of the lymphoreticular system HIV Hu man Marked Broad LCMV Marked Broad CDV Mouse (adu lt) Dog Marked Broad Rubella Hu man Moderate Specific LCMV Mouse Moderate Specific Measles Hu man Moderate Broad HSV Hu man Mild Specific Vaccinia Hu man Mild Specific FLV Mouse Moderate Broad Fet al infection leadi ng to tolerance Perturbation of cytokine home ostasis and intracellular signalling Viral proteins acting as virocept ors or virokines Supp ressor T lymphocytes Global immunosuppression during measles infection 8 6 4 INDURATION(m) Induration (mm) Induration (size of reaction to tuberculin skin test) 2 0 B A S E R A S H 1 2 3 4 W E E K S A F T E R R A S H Mechanisms of Immunosuppression Virus infection and depletion of immune cells (monocytes, T cells, APCs) Tolerance induction Perturbation of immune response thru secreted viral proteins Virus infection and depletion of immune cells (monocytes, T cells, APCs) HIV infection and CD4 T cell depletion HIV replicates primarily in CD4+ expressing T cells Destruction of T-helper and T-effector cells by direct cytopathic and bystander effects Insidiously, Ag-specific CD4+ T cells appear to be preferentially infected monocytes/macrophages and dendritic cells can also be infected; noncytopathic infection (reservoirs?) HIV-specific CD45RO+ CD4+ T cell CMV-specific CD45RO+ CD4+ T cell Total CD45RO+ CD4+ T cell Douek DC et. al. (2002) HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95 PBMCs from HIV+ patients (2 X 108 cells) Stimulate with overlapping peptide pools that span entire HIV proteome Stimulate with CMV peptides Ag specific clones will be activated when stimulated by cognate peptide, and will upregulate CD69 (activation marker) and secrete IFNg FACS sort for CD3+, CD4+, CD69+, IFNg positive cells Real-time PCR to quantify the amount of HIV DNA present Real-time PCR Taq F Q Primers and probe anneal to target Taq Taq begins to displace 5’ end of probe as extension proceeds Taq 5’ nuclease activity of Taq cleaves off 5’ fluorophore on probe Probe begins to fluoresce as it separates fromQuencher, fluorescence builts up as PCR products accumulate Virus infection and depletion of immune cells (monocytes, T cells, APCs) HIV infection and CD4 T cell depletion HIV replicates primarily CD4+ expressing T cells Destruction of T-helper and T-effector cells by direct cytopathic and bystander effects Insidiously, Ag-specific CD4+ T cells appear to be preferentially infected Mechanism?? HIV-specific CD45RO+ CD4+ T cell monocytes/macrophages and dendritic cells can also be infected; noncytopathic infection (reservoirs?) CMV-specific CD45RO+ CD4+ T cell Total CD45RO+ CD4+ T cell Douek DC et. al. (2002) HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95 HIV Direct transfer of virus captured on cs via DC-SIGN? MHC Class II CD4 TCR DC-SIGN: A Conduit for Transfer of HIV to Lymphoid Organs? Geijtenbeek, T.B.H. et al, Cell 100: 594 Virus infection and depletion of immune cells (monocytes, T cells, APCs) Alveolar macs LCMV and APCs Clone 13 preferentially infects and destroys macrophages and DCs APCs are destroyed first before cognate antigen presentation can take place Armstrong strain is immunogenic and thus wellcontrolled by responding immune system Immune clearance Virus titer Virus titer Virus titer Virus infection and depletion of immune cells (monocytes, T cells, APCs) LCMV and APCs Clone 13 preferentially infects and destroys macrophages and DCs Armstrong strain is immunogenic and thus wellcontrolled by responding immune system Clone 13 strain of LCMV is globally immunosuppressive Tolerance induction when newborns are infected CTL response (lytic un its ) on target cells infe cted with Infection with fection with clone 13 at age Herpes simplex Influenza Vaccinia LCMV Immuno supp ression Not infected ot infected 389 586 4000 16246 None 1 day 378 628 5769 0 LCMVspecific 0 56 159 0 Broad one 13 at age < 1 day 6 weeks weeks Mechanisms of Immunosuppression Virus infection and depletion of immune cells (monocytes, T cells, APCs) Tolerance induction Perturbation of immune response thru secreted viral proteins Tolerance Induction: Virus as Self Tolerance Required for prevention of autoimmunity (we are tolerant of self-antigens) Thymic deletion of “forbidden” clones; T-cell clones are “educated” in the thymus during development Peripheral exhaustion of “forbidden” clones Characteristic of tolerogenic viral infections Most easily induced during fetal development Most readily induced if viral replication produces high levels of viral antigens Virus specific Leads to viral persistence LCMV and tolerogenic Infections Neonatal mice infected with LCMV (clone 13) are tolerant only to LCMV but responds well to other viruses Thymic deletion: LCMV infects immature thymocytes; T cells being educated in the presence of LCMV expressing T cells in the thymus are thus tolerant (LCMV reactive clones are deleted) CTL response (lytic units) on target cells infected with Infection with fection with clone 13 at age Herpes simplex Influenza Vaccinia LCMV Immunosuppression Not infected ot infected 389 586 4000 16246 None 1 day 378 628 5769 0 LCMVspecific 6 weeks weeks 0 56 159 0 Broad one 13 at age < 1 day LCMV and tolerogenic Infections Peripheral exhaustion of reactive clones CTL induction is faster with a higher viral innoculum but vast majority of activated CTLs apoptose rather than self-renew and differentiate into effector cells Virus-induced high-dose tolerance produces virus-specific immunosuppression LCMV-tolerized mice still capable of raising vaccinia-specific response CTL activity, lytic units (X 1000) per spleen on targe t cells infected with LCMV CMV (Docile strain) Docile strain) 102 2PFU PFU ot infected Not Infected 107 7PFU PFU 7 PFU 107 PFU LCMV Vaccinia Virus Uninfected Not infected Infected 228 ND ND 31 ND 0.3 Not infected Infected <0.2 <0.2 ND 26 <0.2 0.1 Vaccinia virus Mechanisms of Immunosuppression Virus infection and depletion of immune cells (monocytes, T cells, APCs) Tolerance induction Perturbation of immune response thru secreted viral proteins Viral Proteins that perturb immune responses Effect on lymphocyte proliferation Immunosuppressive proteins encoded by viruses that reduce T cell proliferation (eg. P15e of some retroviral envelopes) Effect on complement Accessory proteins encoded by HSV (gC and gI) can prevent complement activation by binding Fc porion of anti-HSV (gC) antibodies Severity of virus-indu ced lesions Strain of HSV Wildtype gI-negative gE-negative Epith elium of cornea Corneal stroma Brain (encephalitis) Severe moderat e minimal moderat e none none fatal none none Viral Proteins that perturb immune responses Effect on lymphocyte proliferation Immunosuppressive proteins encoded by viruses that reduce T cell proliferation (eg. P15e of some retroviral envelopes) Effect on complement Down-regulation of MHC Class I MHC Class I essential for Ag presentation of viral-derived peptides MHC Class I down-regulation prevents CTL lysis of virus infected cells Different mechanisms: HIVnef, Adeno E1A, E3 Viral Proteins that perturb immune responses Immunosuppressive proteins encoded by viruses that reduce T cell proliferation (eg. P15e of some retroviral envelopes) Effect on complement Down-regulation of MHC Class I Interference with Cytokine functions IFN-g receptor homology (B8R) encoded by poxviruses blunt effects of IFN-g secreted by Agspecific T cells Anti-viral effects of IFNg can be abrogated by B8R: vesicualr virus titer in the presence of IFNg +/- B8R 1 0 0 8 0 6 0 4 0 PFU(%CONTROL) Effect on lymphocyte proliferation B 8 R B A C U L O C O N T R O L 2 0 0 1 1 0 0 8 0 2 3 4 5 4 5 V A C C I N I A C O W P O X C O N T R O L 6 0 4 0 PFU(%CONTROL) 2 0 0 1 2 3 M E D I U M ( L O G C E L L E Q U I V A L E N T S ) 1 0 Controls: Culture Supernatants from (1) Recombinant vaccinia (DB8R8) (2) Wt Vaccinia + anti-B8R Ab (3) Mock-infected (B8R) 125I-IFN-g IFN-gR homolog IFN-gR Measure amt of radioactivity bound to cells In the presence or absence of IFN-gR homologs Chemical Cross-linking 125I-IFN-g + (B8R) 125I-IFN-g LCMV Morbilliviruses: Canine Distemper Virus Acute febrile & fatal disease in dogs Encephalitis, pneumonitis, gasteroeteritis Immunosuppression results in inability to control bacterial/fungal superinfection CDV infects lymphocytes, monocytes resulting in severe leukopenia Loss of lymphoproliferative response Loss of re-call antigen response Retroviruses: MLV/MAIDS CTL response (% 51Cr release) Animal Viruses Associated with Immunosupression Human Viruses Associated with Immunosupression Measles Lack of lymphoproliferative response 50% decrease in T-cell count Few monocytes/lymphocytes are actually infected: immunosuppression must be indirect Envelope glycoproteins (F and G proteins) alone can inhibit T-cell proliferative response Binding of F/G complex to measles virus receptor (CD46) may trigger intracellular signaling cascades leading to inhibition Stimulation Index T-cells PHA proliferation Expt. and controls? (DNA synthesis) Rubella HV/SIV 3H-thymidine Stimulation Index: Amt. Radioactivity (+PHA) Amt. Radioactivity (-PHA)