* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The importance of the immune system
Psychoneuroimmunology wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Duffy antigen system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
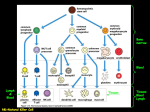
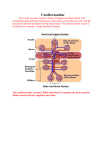
Functional Anatomy of the Adaptive Immune Response Jason Cyster • Describe the principal functions of spleen, lymph nodes, tonsil • Explain how lymphocytes get from the blood into a lymph node • Describe the mechanism of dendritic cell antigen transport • Understand how B cells encounter antigen • Describe changes in effector T cells that permit migration to sites of inflammation Lymphatics (thin walled) One-way valves Lymph is filtered by Lymph Nodes before returning to circulation (liters per day) Lymph contains T and B cells and dendritic cells Anatomy of a Lymph Node afferent lymphatic vessels radial sinus germinal center (secondary follicle) Paracortex (T zone) mainly T-cells capsule subcapsular sinus ] Primary follicles (B zones) Cortex (T+B zone) medullary sinus Filter antigens from the lymph - for recognition by T and B cells - for destruction by macrophages to prevent systemic spread medulla efferent lymphatic vessel Spleen - A filter of the blood • Two main functions of the spleen carried out in two major regions 1) White-pulp is where immune responses against blood-borne antigens occur 2) Red-pulp is responsible for monitoring and removing old or damaged RBC • Red-pulp consists of thin walled splenic (or venous) sinuses and dense collections of blood cells (including numerous macrophages) that form red-pulp cords (or cords of Billroth) • Blood supply: branches of central arteries open directly into red-pulp cords, adjacent to the splenic sinuses (open circulation) – Released RBC must cross the sinus walls; interendothelial slits are a major mechanical barrier and only the most supple, mechanically resilient RBC survive; old and damaged cells are removed by macrophages Anatomy of the Spleen Follicle Red Pulp (B zone) cord Splenic (venous) sinus White-Pulp cord PALS or T zone (periarterial lymphoid sheath) Pulp vein Capsule Trabecular vein Trabecular artery SECONDARY LYMPHOID ORGANS Splenic White-Pulp Cord Lymph Node lymph fluid Mucosal lymphoid tissue (e.g. Tonsil, Appendix) intestinal contents B cell follicle hev ca blood T cell zone hev red-pulp - filter antigens from body fluids - bring together antigen presenting cells and lymphocytes - help bring together antigen reactive B and T cells Lymphocytes traverse HEVs to enter lymph nodes and then compartmentalize in B cell follicles and T cell zones Follicle or B zone - B cells - FDCs HEV (High Endothelial Venule) T cell zone (paracortex) - T cells - DCs LN section stained with: B cell marker L-selectin ligand The cascade (multistep) model of leukocyte extravasation Selectins • lectins are sugar (carbohydrate)-binding proteins; selectins are a specialized type of lectin that bind appropriately glycosylated membrane proteins • Three types: – L-selectin: restricted to lymphocytes • ligands on lymphoid tissue HEVs – P-selectin: made by platelets and activated (inflammed) endothelium – E-selectin: made by activated (inflammed) endothelium • E- and P-selectin ligands expressed on neutrophils, monocytes, activated T lymphocytes Chemoattractant-Cytokines or “Chemokines” >40, small secreted proteins Four families: C, CC, CXC, CX3C chemokine outside chemokine receptor cytoplasm Cells with the appropriate receptor migrate (chemotax) up chemokine gradient Chemokines also promote cell adhesion to endothelium Lymphoid chemokines – help direct the homeostatic trafficking of cells through lymphoid tissues (e.g. CCR7 / CCL21; CXCR5 / CXCL13) Inflammatory chemokines – induced at sites of inflammation; can be expressed by many cell types; help recruit cells to these sites (e.g. CXCR2 / IL-8; CCR2/MCP1) Integrins • heterodimers of and polypeptide chains • can be in inactive (low affinity for ligand) and active states • intracellular signals can cause ‘inside-out’ signaling in the integrin, converting it from an inactive to an active state • chemokine and antigen receptor signaling can activate integrins • bind extracellular matrix proteins (e.g. binds fibronectin) or transmembrane proteins (e.g. integrin LFA1 binds ICAM1; integrin binds VCAM1) Cascade Model of Lymphocyte Recruitment into Lymph Nodes HEV blood flow STEP 1: Tethering and Rolling: L-Selectinglycosylated HEV ligands STEP 2: Integrin Triggering: Chemokines (e.g. CCR7- CCL21) STEP 3: Firm Adherence: LFA1/ICAM1 STEP 4: Transmigration HEV Lymphoid Tissue Multi-step cascade of lymphocyte migration to site of infection/inflammation: same logic, different ‘area code’ • Inhibitor of integrin (tysabri) in clinical use for treatment of Multiple Sclerosis Schematic view of a lymph node CXCL13 Follicle Paracortex CCL21 Medulla CXCL13 -> CXCR5 CCL21 -> CCR7 B zones produce a B cell attracting chemokine T zone produces T cell and DC attracting chemokines DCs migrate from periphery to lymphoid organ T zone bearing Ag • immature ‘sentinel’ DCs are present in most tissues, continually sampling their microenvironment for antigen – by pinocytosis, phagocytosis and engulfment of apoptotic cells • detection of ‘danger signals’ (e.g. LPS, dsRNA, bacterial DNA, necrotic cells, TNF, IL-1) causes the cells to mature – decrease adhesion to local tissue cells (e.g. keratinocytes) – increase expression of receptors (CCR7) for chemokines made by lymphatic endothelial cells and lymphoid organ T zones – upregulate MHC and costimulatory molecules • migrate into lymphoid T zone • present antigen to T cells Immature (sentinel) DCs in tracheal epithelium longitudinal section tangential section DCs migrate from periphery to lymphoid organ T zone bearing antigen Skin draining Lymph Node Follicle T zone DC LC = Langerhans’ Cell, the immature DC of the skin B cell antigen encounter antigen lymph fluid Sinus Macrophage FDC hev • B cells bind intact antigen through their surface Ig / B cell receptor (BCR) • Antigen that enters via blood or lymph reaches the follicle and can be captured directly by B cells • Follicular dendritic cells (FDCs – tissue resident cells related to fibroblasts) can display antigen on their surface in an intact form for long periods Lymph node egress occurs in response to a circulatory lipid (sphingosine-1-phosphate, S1P) MEDULLARY SINUSES S1P Diagram courtesy of Ted Yednock -Lymphocytes express a receptor (S1PR1) for S1P - Egress involves migrating to S1P that is high in blood/lymph and low in the tissue Lymphocytes express S1PR1 and exit lymphoid organs in response to S1P lymph node, Peyer’s patch thymus, spleen S1PR1 S1PR1 S1P lyase S1P lyase Immunosuppressant RBC S1P blood S1P FTY720 efferent lymph • S1PR1 is required for T cell egress from thymus and for T and B cell egress from spleen, lymph nodes, tonsil • Activated lymphocytes transiently down-regulate S1PR1 and are retained in the responding lymphoid tissue until they become effectors • FTY720 (Fingolimod) inhibits egress and is in clinical development as an immunosuppressant (FDA approved in 2010 for treatment of multiple sclerosis) Effector T cell Trafficking • Activated T cells exit lymphoid tissue -> circulation – upregulate S1PR1 – ability to re-enter lymphoid tissue is reduced (decrease in CCR7, L-selectin) • Increased ability to enter inflammed tissue due to increased expression of: – ligands for E- and P- selectins – receptors for inflammatory chemokines (e.g. CXCR3) – adhesion molecules (e.g. integrin )