* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Implications of PGD in the Halakhic and
Fetal origins hypothesis wikipedia , lookup
SNP genotyping wikipedia , lookup
Gene therapy wikipedia , lookup
Genetic code wikipedia , lookup
Heritability of IQ wikipedia , lookup
Genetic drift wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Behavioural genetics wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Point mutation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genealogical DNA test wikipedia , lookup
DNA paternity testing wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Population genetics wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Human genetic variation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Genetic engineering wikipedia , lookup
Medical genetics wikipedia , lookup
Genetic engineering in science fiction wikipedia , lookup
Public health genomics wikipedia , lookup
Genome (book) wikipedia , lookup
Microevolution wikipedia , lookup
Genetic testing wikipedia , lookup
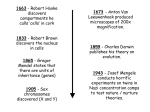
The Implications of PGD in the Halakhic and Secular Spheres Can we use modern technology to change our genetic fate? Shira Levie Preimplantation genetic diagnosis is one of the most revolutionary medical advances in recent years. It has altered the way people view the risk of conceiving children with birth defects. Preimplantation genetic diagnosis (PGD) is an early form of genetic testing where genetic defects in embryos, conceived through in vitro fertilization, are analyzed before implantation in the uterus. This offers couples at risk of genetic diseases the chance to have a healthy child, without facing termination of pregnancy.1 The Oxford University Press Medical Dictionary expands on this definition adding that PGD is prenatal genetic diagnosis, extended into the earliest stages of embryonic development, before implantation occurs. This definition only scrapes the surface of PGD and the implications that come along with it. To fully understand the scientific, as well as ethical problems that arise in PGD, it is necessary to examine every stage and facet involved in preimplantation genetic screening. The first step in developing preimplantation genetic testing began in 1968. It was that year that researchers Robert Edwards and David Gardner first reported the success of sexing rabbit embryos. This marked an important step in the process of developing preimplantation genetic diagnosis. Scientists were then able to slowly grasp how to obtain information from the genetic makeup of certain embryos.2 Ten years later, with the development of in vitro fertilization, PGD took on a new role. Sermon K. Current concepts in Preimplantation genetic diagnosis (PGD): a molecular biologist's view. Hum Reprod Update 2002;22:312-8. 2 Harper JC. Introduction. In: Harper JC, Delhanty JDA, Handyside AH, eds. Preimplantation Genetic Diagnosis. London, UK: John Wiley & Sons; 2001:3-12. 1 This new technology was embraced by the medical community, allowing prospective parents to select genetically healthy embryos for implantation. Through these new technologies, the field of preimplantation genetics has grown tremendously. As of 2006, more than 15,000 PGD cycles had been reported.3 PGD is currently available for most known genetic mutations, such as Aneuploidies, Cystic fibrosis, Hemophilia A and B,and Tay Sachs disease.4 Since only unaffected embryos are selected through PGD to be implanted to the uterus it provides a preferable alternative to current prenatal diagnostic procedures which occurs at the beginning of pregnancy and can be performed as late as nine weeks into the pregnancy.5 Since the baby is already beginning to form by this stage of the pregnancy, the test is frequently followed by the difficult decision of pregnancy termination if the results indicate an unhealthy fetus. Additionally, prenatal testing results an 8% higher spontaneous miscarriage possibility then on those fetuses not tested. 6 Since preimplantation genetic diagnosis tests are done on embryos resulting from in vitro fertilization and only the healthy ones are implanted, it avoids the issue of termination of pregnancy. With these advantages, this new method has quickly gained popularity among prospective parents. While this technique is widely accepted and many depend on its results, there is still a long way to go for PGD. Reports of the identification of genetic changes linked to various diseases are published almost 3 SART CORS Web site. Available at https://www.sartcorsonline.com/. International Working Group on Preimplantation Genetics, International Congress of Human Genetics.Preimplantation Genetic Diagnosis: Experience of Three Thousand Cycles. Report of the 11th Annual Meeting of International Working Group on Preimplantation Genetics, in association with 10th International Congress of Human Genetics. Vienna, Austria: May 2001:[Full Text]. 5 Amniocentesis and chorionic villus sampling for prenatal diagnosis (Review). By Alfirevic Z, Mujezinovic F, Sundberg K at The Cochrane Collaboration, 2009 6 ibid 4 weekly.7 Scientists are constantly working to improve the accuracy of these tests and the techniques used to obtain the results. The main attraction of preimplantation genetics to prospective parents is the prevention of birth defects in their offspring. For all pregnancies, the baseline risk of some type of birth defect is 3 to 4%.8 Since the severity of such defects varies widely as a result of inheritance, occurrence of spontaneous mutations, and environmental influence, it is hard to pinpoint a set of guidelines for couples in order to justify having PGD preformed on their embryo.9 One type of couple that would be a candidate for PGD testing is a couple that has a history of known genetic conditions in their immidiate family or who have given birth to a child with a genetic disorder. This could be a disease caused by a single gene defect, a monogenic disease, or chromosomal abnormality in the offspring, due to a balanced chromosomal rearrangement in one of the partners. These types of couples are offered guidance by The American Congress of Obstetricians and Gynecologists (ACOG.) It recommends that women be offered information about genetic risks, and therefore can decide if they should test their embryos using PGD. 10 While couples who have previous experience within their immediate family with genetic defects are more inclined to use PGD, a family history of genetic disorders is not required for genetic screening. Genetic testing may be carried out when there is suspicion that an individual is at increased risk because of extended 7 Molina B Dayal, MD, MPH Associate Professor, Medical Director of Egg Donation Program, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, Medical Faculty Associates, George Washington University School of Medicine 8 McKusick–Nathans Institute of Genetic Medicine, Johns Hopkins Medical Institutions, Baltimore; Department of Obstetrics and Gynecology 9 McKusick–Nathans Institute of Genetic Medicine, Johns Hopkins Medical Institutions, Baltimore; Department of Obstetrics and Gynecology 10 Ibid family history or because of a positive result on a biochemical screening test.11 Additionally, some couples use this technology for social reasons in order to select gender and other social traits. While not recommended, this is an unfortunate reality of this new technology. This has sparked an ethical debate within the medical community. Preimplantation genetic diagnosis can be carried out at different stages of embryonic development. There are three methods that can be used to obtain genetic material in order to test for a deficiency in a potential embryo. All three methods have pros and cons and each methodology utilizes a different stage of the embryonic development. The process of egg production, called oogenesis, begins in the female fetus. Each woman is born with a certain number of cells capable of becoming an egg. These cells go through oogenesis to produce oocytes. After maturing, the oocyte divides into two new cells of unequal size: a relatively large oocyte and a minute polar body. A second stage of division occurs which produces a second polar body and a large ootid (mature egg cell.) All cells contain the maternal DNA, but the polar bodies eventually die leaving the mature egg cell to be fertilized. When an egg is fertilized by a sperm cell it creates a zygote containing half of the genetic information from each parent. This zygote then undergoes the cleavage stage where it divides rapidly, first becoming a ball containing four cells, then an eight cell ball called the cleavage stage. After many more rounds of cell division the developing embryo becomes a blastomere containing hundreds of cells. The process continues and the cells divide forming organs and limbs, and ultimately a child. 11 Ibid The first method for carrying out PGD examines the genetic material within the polar bodies, the by-products of the first and second stages of division.12 These cells are exclusively made up of the mother’s DNA. This method can be used in case of maternally derived dominant mutations, aneuploidy and translocations which are chromosome defects caused by the rearrangements of parts between non similar pairs of DNA. The first and second polar bodies can be analyzed to determine the presence of maternal genetic contributions (i.e., X-linked diseases and autosomal dominant diseases), including carrier states for Duchenne's muscular dystrophy, incontinentia pigmenti, and neurofibromatosis type. 13 While this method is advantageous as the polar bodies are considered a waste of nuclear material after meiotic division and do not have any physiological role to play in the development of embryos, it does present some difficulties. The polar bodies cannot be used when paternally derived genetic information is critical for the diagnosis, such as paternally derived mutations, translocations, and aneuploidy.14 Additionally this technique presents a problem as many chromosomal abnormalities arise after fertilization. Polar body testing can prove to be inaccurate regarding the actual health of the baby. Nevertheless, it is a widely accepted practice to screen for diseases as it yields improved pregnancy outcomes by detecting maternal genetic abnormalities in eggs, including meiotic errors that result in aneuploidy. The most widely used approach for obtaining genetic material is testing the individual embryonic cells from the cleavage state of the embryo. These cells, called blastomeres, are obtained on the third day after in vitro fertilization and are removed 12 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 13 McKusick–Nathans Institute of Genetic Medicine, Johns Hopkins Medical Institutions, Baltimore Department of Obstetrics and Gynecology 14 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 through a hole created in the zona pellucida,15 the strong membrane that forms around an ovum as it develops in the ovary. After testing, the embryos can be transferred into the uterus on day four or five. This allows enough time for genetic analysis to be performed and for the implantation of genetically normal embryos, which have now progressed through the blastocyst stage.16 The advantage of this method is that it contains both the maternal and paternal genes. However, it must be taken into consideration that there is a limited amount of cells available for use in this stage. That can limit the accuracy of results as well as the development of the fetus. Another approach is to use the non-embryo forming cells (trophoectoderm cells) of the embryos on day 5 of development.17 In this case, the cells that are taken will not become part of the baby, rather they will grow into what will become the placenta. The main advantage of this method is that many cells are available to be genetically analyzed, unlike the blastomere biopsy, where very few cells are available. These cells also are obtained by the culturing of the zygote until they reach the blastocyst stage and then Zona drilling to remove cells that can be used for biopsy. The major limitation of this approach is the requirement that a sufficient number of embryos reach the blastocyst stage in vitro, thus giving a sufficient number of cells. There is a very brief opportunity for genetic assessment at this stage as the embryos need to be implanted quickly after assessment. 18 This time constraint leaves a very small window for testing and can prove to be inaccurate. Lastly, the TE cells may not reflect the true genetic features of the cells. 15 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 16 Ibid 17 Ibid 18 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 After the polar bodies or cells are obtained, there are different methods that are used for screening for genetic abnormalities. Each screening involves tests for the most common mutations and specific diseases in particular populations.19 The three methods that are commonly used to carry out testing are polymerase Chain reaction (PCR), fluorescence in situ hybridization (FISH), and single-nucleotide polymorphism (SNP.) The screening method employed will depend on the type of genetic disorder suspected based on the knowledge of each specific case. In all cases the embryo's health is not effected by the tests if preformed correctly. Polymerase Chain Reaction (PCR.) is used for the diagnosis of single gene defects. This test has a 97% accuracy rate, making it a fairly reliable diagnostic test.20 Recent advances in DNA sequencing and bioinformatics have led to an approach that identifies carriers of known mutations that cause more than 400 recessive genetic diseases. These include both dominant and recessive disorders such as cystic fibrosis, spinal muscular atrophy, myotonic dystrophy, Huntington disease, and Marfan syndrome. This test makes many copies of the region of interest in the DNA of a biopsied cell, allowing scientists to study the DNA and determine the health of the selected embryo. In this test, the DNA is immersed in a solution containing the DNA polymerase enzyme, unattached nucleotide bases, and primers. The solution is heated to break the bonds between the strands of the DNA. When the solution cools, the primers bind to the separated strands, and the DNA polymerase quickly builds new strands by joining the free nucleotide bases to the primers. Further repetitions of the 19 McKusick–Nathans Institute of Genetic Medicine, Johns Hopkins Medical Institutions, Baltimore Department of Obstetrics and Gynecology 20 Munne S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, et al. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod Biomed Online.2003;7:91–97. process can produce billions of copies of a small piece of DNA in several hours.21 This test can be performed on a polar body, cleavage, or TE cell. PCR is a fast and convenient way to test DNA. However, it requires sufficient amounts of an uncontaminated, high-quality sample of DNA, which is sometimes difficult to obtain from a single cell such as a polar body or blastomere. The laboratory in which PCR is being carried out must be strictly controlled to avoid the contamination of the tested material. The laboratory technicians must be exceptionally well-trained to avoid all types of foreign, interfering factors. In addition, allele dropout is a serious complication. The phenomenon known as allele dropout presents one of the most common problems for PCR.22 Both cleavage cells and balstomeres contain two copies of every gene, each called an allele. Either one or both of the alleles may be defective. Allele dropout refers to the preferential amplification of one allele over another during the PCR process and is mainly a problem for PGD of dominant disorders or when two different mutations are carried for a recessive disorder and only one mutation is being analyzed. Therefore, all of the possible genetic makeups of the embryonic cells are not represented. This results in a 3% likelihood of inaccuracy. These inaccuracies in PCR can result in misdiagnoses, harming embryos as they are handled or the discarding of a normal embryo. This risk of using PCR is easily overlooked as the success rate is so high. It may be noted however that a polar body contains a single copy of the DNA (haploid) and the cell biopsied at all other 21 Treff NR, Tao X, Lonczak A, Su J, Taylor D, Scott RT., Jr Four hour 24 chromosome aneuploidy screening using high throughput PCR SNP allele ratio analyses. Fertil Steril. 2009 22 Scott RT, Jr, Tao X, Taylor D, Ferry K, Treff N. A prospective randomized controlled trial demonstrating significantly increased clinical pregnancy rates following 24 chromosome aneuploidy screening: biopsy and analysis on day 5 with fresh transfer. Fertil Steril. 2010 stages have double copies of the DNA (diploid). Therefore using a polar body is difficult for PCR, as only a single cell is the source of DNA.23 The DNA must be amplified many times in order to have enough material to analyze. Taking that into consideration, as well as the issue of allele dropout, PCR is still a very accurate way of testing and many doctors turn to this method as a way to detect mutation. Fluorescence in situ hybridization (FISH) is another method used in PGD. FISH is used for the determination of chromosomal abnormalities such as aneuploidy and the sex of an embryo to better diagnose X-linked diseases. Aneuploidy refers to an abnormal number of chromosomes, which causes birth defects such as down syndrome. Aneuploidy screening is by far the most common indication for PGD. Since an abnormal chromosome number can be the cause of low sucess in implanting embryos as well as recurring miscarriges of unidentified causes, PGD has been used to increase the success rate of full term pregnancies.24 The way FISH is carried out is by using probes (ie, small fragments of DNA that match the chromosomes being analyzed) and binding them to a certain chromosome. Each probe is labeled with a different fluorescent dye. These fluorescent probes are applied to the cell biopsy sample and attach to the specific chromosomes. They can be observed under a fluorescent microscope. The number of chromosomes of each type (color) present in that cell is counted. The geneticist can then distinguish normal cells from abnormal cells. The common probes are used to detect abnormal chromosomes for chromosomes X, Y, 13, 18, and 21. The number of chromosomes tested in a single FISH is limited by the number of fluorescent probed 23 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 24 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 available. However, mixing of colors, several rounds of FISH on the same cell, and comparative genomic hybridization (CGH), enhance the possibility of detecting the abnormalities in several chromosomes/regions.25 One of the key limitations of FISH based preimplantation aneuploidy screening is the inability to simultaneously evaluate all 24 chromosomes found in human cells (chromosome 1–22, X and Y).This limits the number of irregularities that FISH can screen for. FISH would not be the preferred mode of testing if all chromosomes need to be analyzed. Additionally, in an evaluation of FISH, results were obtained indicating that FISH gives inconsistent results.26 However, FISH offers accurate results in detecting Aneuploidy screening in women of advanced maternal age, aneuploidy screening for male infertility, identification of sex in X-linked diseases, and recurrent miscarriages caused by parental translocations. FISH can be used for these detections in which it is accurate. When FISH is the screening technique used, false results may be generated by failure of the probes to hybridize, poor signal intensity, split or fused signals. As only a single cell is analyzed, mosaicism, the evidence that mosaic embryos are able to stop the reproduction of abnormal cells and continue to develop into a healthy embryo, is not accurately detected by this technique. Mosaicism poses a problem to PGD as it can obtain false results and cause the discarding of many healthy embryos. This is especially important, as there may be a trisomy rescue and embryos that are diagnosed to have a trisomy may eventually develop into a fetus with normal chromosomal content. It is estimated that misdiagnosis in aneuploidy screening after 25 Adiga S K, Kalthur G, Kumar P, Girisha K M. Preimplantation diagnosis of genetic diseases. J Postgrad Med 2010;56:317-20 26 Fiegler H, Geigl JB, Langer S, Rigler D, Porter K, Unger K, et al. High resolution array-CGH analysis of single cells. Nucleic Acids Res. 2007; the biopsy of one blastomere is 7%, with 6% due to mosaicism. 27 Mosaicism is the idea that many embryos identified as aneuploid will survive and return to normal after correcting the defect in a cell.28 As there is a high level of chromosomal mosaicism in the cleavage stages of embryonic development, which can confound the interpretation of data or demand follow-up analysis, and because contemporary FISH methods do not capture the full complement of chromosome material, the extent to which preimplantation genetic screening is useful in improving pregnancy rates and outcomes is debated.29 This is a downside to this testing as the embryo can correct itself, causing the discarding of potentially healthy embryos. This also provides the risk of false test results in a circumstance in which one cell in the embryo is healthy and the rest are defective, and the healthy one is tested, then false results about the health of the embryo will be obtained. The most recent developments in Preimplantation genetics has created a new screening process using single nucleotide polymorphisms, or SNPs. These are DNA sequence variations that occur when a single nucleotide in a strand of DNA is altered. According to the Human Genome Project for a variation to be considered a SNP, it must occur in at least 1% of the population. SNPs, which make up about 90% of all human genetic variation, occur every 100 to 300 bases along the 3-billion-base human genome. SNPs can occur in coding (gene) and noncoding regions of the genome. Many SNPs have no effect on cell function, but they have been correlated with an 27 Munné S, Magli C, Bahçe M, Fung J, Legator M, Morrison L, et al. Preimplantation diagnosis of the aneuploidies most commonly found in spontaneous abortions and live births: X, Y, 13, 14, 15, 16, 18, 21, 22. Prenat Diagn 1998;18:1459-66 28 Rius M, Daina G, Obradors A, Ramos L, Velilla E, Fernández S, et al. Comprehensive embryo analysis of advanced maternal age–related aneuploidies and mosaicism by short comparative genomic hybridization. Fertil Steril. 2011 29 McKusick–Nathans Institute of Genetic Medicine, Johns Hopkins Medical Institutions, Baltimore Department of Obstetrics and Gynecology increased probability of developing diseases such as diabetes, vascular disease, and some forms of mental illness. These associations are difficult to establish with conventional gene-hunting methods because a single altered gene may make only a small contribution to the disease. In order to test for these SNP’s high-density oligonucleotide, SNP arrays are used. In this form of testing, hundreds of thousands of probes of DNA from a zygote are arrayed on a small chip, allowing for many SNPs to be investigated simultaneously. Because SNP alleles only differ in one nucleotide and because it is difficult to achieve optimal hybridization conditions for all probes on the array, the target DNA has the potential to hybridize to mismatched probes. This is addressed somewhat by using several unnecessary probes to hybridize to each SNP. By comparing the differential amount of hybridization of the target DNA to each of these probes, it is possible to determine specific homozygous and heterozygous alleles. The use of SNP arrays proves to be beneficial in many ways. A study was done proving that SNP array data is able to detect mosaicism better than the FISH method. 30 This is very important as mosaicism is a major contributor to the error rate in PGD. It also may be able to detect aneuploidy among all 24 chromosomes, which has not been achieved by other methods of testing. While SNP proves to be an effective and worthy method, it also comes with complications. This type of test is very expensive and the results may be inconsistent because of overlapping probes that sometimes are considered independent, presenting errors in the data.31 SNP array analysis is fairly new to the world of PGD and the technology is still being modified 30 Fiegler H, Geigl JB, Langer S, Rigler D, Porter K, Unger K, et al. High resolution array-CGH analysis of single cells. Nucleic Acids Res. 2007; 31 Caignec C, Spits C, Sermon K, Rycke M, Thienpont B, Debrock S, et al. Single-cell chromosomal imbalances detection by array CGH. Nucleic Acids Res. 2006 to be as accurate as possible. It is an alternative to PCR and FISH and is expected to become the quintessential way of performing PGD in the years to come. In addition to the limitations pertaining to each test individually, one limitation that is present for all types of testing is that it is not always possible to predict the severity of a clinical condition on the basis of a genotype (only the genetic makeup of the embryo). A laboratory result may be flawless, but the identified genetic variation may not be known to cause a disease (i.e., it is a variant of uncertain significance). Alternatively, the discovered mutation or variant in a known disease gene may not reliably correlate with a phenotype, what actually becomes of the zygote, because of the influence of modifiers, which can be genetic, epigenetic, or environmental.32 This can result into the discarding of healthy embryos or the implantation of unhealthy ones. This presents not only a medical, but also an ethical and religious problem. Considering the limitations, methods, and results of PGD, one must now approach this type of test from both an ethical and religious standpoint. When assessing this problem, it is necessary to be mindful of the views of society as well as the approaches of the Jewish sages and modern Halakhic authorities. Preimplantation Genetic Diagnosis presents many dilemmas in modern medical ethics, all which are considered by Rabbis in order to make decisions. Since Jews marry within their ethnicity they have a much higher rate of many gene mutations, most notably TaySachs, a lethal disease common in the Ashkenazi Jewish community. Another common gene in the Jewish community is the BRCA gene, which, when found, is an early indicator of breast cancer as a woman ages. These genetic mutations cause PGD to be an emerging topic as many Jewish couples wonder if preimplantation testing is 32 McKusick–Nathans Institute of Genetic Medicine, Johns Hopkins Medical Institutions, Baltimore Department of Obstetrics and Gynecology their best option to obtain a healthy embryo. The Rabbis must consider many Jewish laws when deciding what is halakhically permissible when preforming PGD on an embryo. The initial question regarding PGD and halakha is if halakha even permits PGD for a couple conceiving. As previously mentioned, PGD is a preferred method of screening over prenatal genetic screening, as it does not present the ethical problem of termination of pregnancy. The issue of abortion is a charged topic, debated in politics as well as in religion and medical ethics. On one hand is the issue of the sanctity of life and the concern for the right to life of the yet unborn. On the other hand, government regulations of abortion are seen as a violation of privacy and a woman’s right to choose whether or not to see a pregnancy to term. This dilemma is fueled by the disagreement over the timeframe that a fetus is considered a life and the power that a mother has over the life of her potential child. From the standpoint of Jewish Law, up until forty days, the fetus is considered maya b’alma (mere water).33 Until the unborn baby’s head emerges, if causing the mother harm, the fetus is considered a pursuer (rodef). The mother’s life can be preserved through self-defense even if resulting in fetal death.34 Therefore, abortion is generally reserved only for cases involving danger to the mother. However, if the threat is a fetal abnormality, R. Feinstein prohibits abortion and rules that prenatal birth defect detection with the possibility of abortion is not permissible. He derives this from the source in the Seven Laws of Noah which states cases of capital homicide. The Talmud’s rendition of Genesis 9:5, which describes the prohibition of killing says “if a person shed the blood if a man within a man, he shall be killed.” the 33 34 Yevamot 69b, Nida 30a, Rashi Nidda 30a Talmud Bavli Mishnah ohalot 7:6 Talmud proceeds to ask, “and who is a man within a man? This is the fetus within the womb of the mother,” This verse thus prohibits the killing of a fetus within the mother, ruling that abortion is prohibited on a biblical level. Most halakhic authorities agree on this ruling. If abortion is not permitted, a question then arises regarding whether one is able to discard the embryos that were created but deemed unhealthy to implant by way of FISH, SNP, or PCR. The Jewish debate over this matter is fueled by the forbidding of one to waste potential life, as stated in Gen. 38:9-10 “Zera Levatala.” 35 Judaism sees much value in every potential life, extending to not wasting sperm. What does this come to imply about PGD and discarding affected embryos? While allowing IVF, Rabbi Feinstein prohibits embryo discarding in this situation, since it eliminates potential life. However, many other Rabbis such as Rabbi Lichtenstein disagree, because producing healthy children is intended through consideration of parental anguish. The Committee on Medical Ethics of the Federation of Jewish Philanthropies of New York concluded that as the embryo was in a test tube environment in which viability is unattainable, it is not considered human life and can be discarded.36 This is because it is not in a setting where it can flourish and become a human, therefore, since removed from the uterus, it is not considered a potential life. With most Rabbis agreeing that it is halakhically permissible to discard defective embryos, thus deeming the actual tests halakhically permitted, it is necessary to explore if the test’s aims are permitted by halakha. One could challenge whether it is the place of man to intervene and assert control in God’s world. Is man 35 Genesis 38:9-10, Rashi Genesis 38:7, supra, note 56; Rambam, Hilchot Issurei Biah 21:18, supra, note 22; Niddah 13a, 13b, supra, note 21 36 Feldman, D. M. & Rosner, F. (1984). Compendium on Medical Ethics. New York: Federation of Jewish Philanthropies of New York, 28 allowed to interfere with nature and create a genetically modified human? From a secular ethical standpoint,the problem is widely debated and is the source of a set of codes established by the Human Fertilization Embryology Authority, or HFEA, in the United Kingdom. They establish a set of certain diseases and chromosomal abnormalities that an embryo can be screened for, thus instituting guidelines for the extent to which man can play a role in the making of nature’s creation. These codes proved controversial, as many passionately advocated for one extreme or the other, namely, that PGD should always, or never be allowed. This debate, in the secular world, is about interfering with nature. From the Jewish perspective, one is not only tampering with nature, but with God. The Jewish world was shaken by the introduction of PGD, as the question of playing God became a cause célèbre. There is the belief that mankind should play its part in God’s plan, using world resources to continue God’s creation according to the law of tikun olam; healing the world.37 Therefore, we allow physicians to practice and heal, though they are intervening with God’s hand in the health of man. 38 However, a medical procedure that presents no chance of healing violates the Jewish decree against tampering with God’s creation. PGD does not heal, it merely chooses one embryo over another and, therefore, is a valid basis for a Jewish prohibition. There seems to be a lack of halakhic consensus in this area. Many authorities are yet to come to a conclusive ruling, however, consideration of the issues individually assists many Rabbis in deciding whether PGD is halakhically permissible. 37 Genesis 1:26, supra, note 56; Buber, S. (1925). Midrash Shemuel. Vilna: Romm, section 4 38 Sh’mot, 21:18-9, Vayikra 19:16, Devarim 22:1,3, supra, note 56; Bava Kama 85a; Brakhot 60a; Sanhedrin 73a; supra, note 21 Shulkhan Arukh, Yoreh Deah, 336:1, in Steinberg, A. & Rosner, F. (2003) Encyclopedia of Jewish Medical Ethics. Jerusalem: Feldheim, 101 Many Poskim maintain that one can perform genetic testing if the embryo has a ‘serious or significant’ defect. This makes it the job of the Rabbis to decide what is considered worthy of us interfering with God’s plan. The World Health Organization originally defined serious disease in terms of etiology and process, into which disability was subsumed causing impairments, disabilities, and handicap. This would come to include the lethal disease Tay- Sachs. An alternative definition to a ‘serious condition’ used by many contemporary social organizations is that it is the disadvantage of restriction that excludes them from mainstream social activities. This may include Trisomy 21, or the detection of the BRCA gene. The practical difference between these two definitions is that according to the World Health Organization the embryo should only be prevented from being implanted if the child will have a mutation causing severe physical and mental disabilities, lessening quality of life, or in extreme cases, death. In contrast, according to the definition used by many social organizations, if an embryo has any sort of defect that might slightly impair quality of life in some way, the embryo should not be implanted. Should ‘serious’ or ‘significant’ be the criteria used by Poskim? It is difficult to draw a non-arbitrary line between severe and non-severe, it being dependent on the circumstances and dogma. One thing to consider is that Judaism believes in the sanctity of life and upholds the belief that each person is created in God’s image. Each person has spiritual value, regardless of their abilities or lack thereof. There is even a blessing that many say upon seeing a deformed person. The Talmud addresses people with disabilities asking, ‘do you think your blood is redder than his? Perhaps his is redder than yours!,’39 which serves to remind how judgments are made on externalities, lacking knowledge of individuals' true value. Therefore, it is 39 Pesakhim 25b, supra, note 21 questionable as to whether a Rabbi can tell a couple that they can perform PGD, therefore preventing the creation of a mutated child, God's creation. This being considered, it must also be noted that the disabled are excluded from the performance of several mitzvot due to lack of required functionality,40 as one can deduce that PGD is allowed according to halakha in order to maximize the contributions of a Jew into the halakhic world. This can eliminate the line that needs to be drawn between serious and less serious cases, using the potential impairment to participate in Halakha as a way to determine whether or not to implant the embryo. This is supported by Rabbi Yosef Shalom Eliyashuv, an influential posek in Israel today, who has permitted pre-implantation diagnosis on the basis that it is allowed by Halakha. If PGD is indeed halakhically permissible, the debate arises whether or not it is required to test embryos by way of PGD by the Torah obligation to guard his health. One must distinguish if this law is extended to mean to guard his health, and the health of the child he will create. Rabbi Moshe Feinstein favored Tay-Sachs testing and considered the possibility that testing might be a moral obligation. He states that while one may usually ignore a small risk that society finds acceptable not being tested for a disease that can be life threatening is like closing one's eyes to an obvious danger, something that is directly prohibited by the Torah. Therefore, one can conclude that a couple has a moral as well as a religious obligation to use PGD to test for lethal diseases. The extent to which these tests can be used varies. Preimplantation Genetic diagnosis is a revolution in the medical world. Its potenail uses and benefits continue to be developed. However, considering the many methods used to carry out PGD as well as its limitations and error rates there are 40 Outlined in Marx, T. (1993). Thesis: Halakha and Handicap: Jewish Law and Ethics on Disability. Jerusalem: Marx, T. many ethical and religious debates about this new technology. Both in religion and secular ethics, there is no one correct answer as to what extent this technology can be used. In religion, while it does vary by individual case, many influential rabbis agree that PGD is Halakhicly permissible based on many Talmudic and Torah references. This being said it cannot be abused to include social traits that will not affect a person’s contribution to the world of Judaism. PGD, though one of the most controversial medical developments, is an incredibly significant resource for the future of medicine. As it progresses, PGD will become more accurate and inexpensive and ultimately has tremendous potential to change the world.