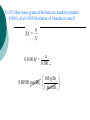* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Solutions
Survey
Document related concepts
Transcript
Solutions Chapter 13 5.0 Chemistry What is a solution Solution: A homogeneous mixture of 2 or more substances in a single physical state. Suspension: Particles are somewhat evenly dispersed, but will settle when at rest. (clay & water) Colloid: Particle size between suspension and solution (milk) Properties of Solutions Small Particles Evenly distributed particles Particles do not separate when at rest. Solubility: The ability to dissolve in another substance. Properties of Solutions Solute: substance that is dissolved Solvent: Substance that does the dissolving Solvent Solute Properties of Solutions Soluble means something can be dissolved in something else. Insoluble means something cannot be dissolved in something else. Solid Solutions Alloys: Solid solutions that contain 2 or more pure substance. Advantages of alloys over pure metals: Stronger Cheaper Resistance from corrosion Lighter Alloys Gaseous Solutions The properties of gaseous solutions depend on the properties of its components. Ex. Air Liquid Solutions Miscible Liquids: 2 liquids that can mix together in any amount Ex. Alcohol and water Liquid Solutions Immiscible Liquids: 2 liquids that cannot mix Ex. Oil and Water Other types of solutions Aqueous Solutions: Solutions with water as the solvent. Because water can dissolve so many things, it is called the universal solvent. Electrolyte: A solution that conducts electricity Tincture: Solutions with alcohol as the solvent. Type of Solution Specific Solute IN Specific Solvent Gas in Gas Air O2 N2 CO2 H2O hydrocarbons Pt H2 O air lemon juice water mercury silver carbon air sugar water carbon iron Gas in Liquid Soda Gas in Solid catalytic converter Liquid in Gas fog Liquid in Liquid Lemonade Liquid in Solid teeth fillings Solid in Gas smoke Solid in Liquid Kool-Aid Solid in Solid steel Concentration and Molarity Concentration: Refers to the amount of solute per given amount of solvent. Dilute Solutions – Has little solute Concentrated Solutions – Has large amount of solute Molarity Molarity – Term used for concentration Molarity = Moles of Solute Liters of Solution D. Molarity: (M) molarity = moles of solute liters of solution M = n V units = mol L Ex #1) What is the molarity of a solution formed by mixing 10.0 g of sulfuric acid with enough water to make 100.0 mL of solution? 10.0 g H 2SO4 1 mol H SO 2 4 = 0.102 mol H 2SO4 98 g H 2SO4 0.102 mol M = 1.02 M 0.1000 L H 2 O Ex #2) How many grams of bromine are needed to prepare 0.500 L of a 0.0100 M solution of bromine in water? n 0.0100 M = n = 0.00500 mol Br2 0.500 L 160 g Br2 0.00500 mol Br2 = 0.800 g Br2 1 mol Br 2 Solubility and the Dissolving Process Saturated: A solution is saturated if it contains as much solute as can possibly be dissolved under existing conditions of temperature and pressure. Unsaturated: Has less than the maximum amount that can be dissolved. Supersaturated: Has more than the maximum amount that can be dissolved. Saturated vs. Unsaturated Saturated vs. Unsaturated Supersaturated Dilutions Formula for Dilutions: M1V1 = M2V2 How a solution forms Process of dissolving takes place at the surface of the solute. Dissolving Process The interaction between the solute and solvent to allow ions to separate is called solvation. This interaction is called hydration when water is the solvent. Dissolving Process Energy is absorbed when the bonds break. (endo) Energy is released when bonds form. (exo) Solubility Solubility is amount of solute that will dissolve in a specific solution under given conditions. See solubility curves Solubility Graph for NaNO3 180 At 20 oC, a saturated solution contains how many grams of NaNO3 in 100 g of water? 90 g Saturated sol’n 170 160 150 140 oC? What is the solubility at 70 135 g/100 g water 120 Solubility ( g/100 g water ) What kind of solution is formed when 90 g NaNO3 is dissolved in 100 g water at 30 oC? unsaturated Supersaturated solution 130 110 100 90 80 70 Unsaturated solution 60 50 40 What kind of solution is formed when 120 g NaNO3 is dissolved in 100 g water at 40 oC? supersaturated 30 20 10 0 0 10 20 30 40 50 60 70 Temperature (deg C) 80 90 100 110 Factors that affect solubility 1) Nature of Solute and Solvent General rule: Like dissolves Like See example 2) Temperature As temperature increases, the solubility of gases and liquids decrease. Gases are less soluble at high temperatures than at low temperatures. Pressure: As pressure increases, the solubility increases. Factors Affecting the Rate of Dissolving 1) Surface Area 2) Stirring 3) Temperature Altering these 3 factors will either increase the frequency of collisions or the energy of collisions between the solute and solvent.