* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CYL110
Bose–Einstein condensate wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Glass transition wikipedia , lookup
Temperature wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Heat transfer physics wikipedia , lookup
Transition state theory wikipedia , lookup
Gibbs paradox wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Degenerate matter wikipedia , lookup
Supercritical fluid wikipedia , lookup
State of matter wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Microplasma wikipedia , lookup
History of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Van der Waals equation wikipedia , lookup
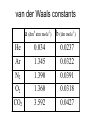
CYL110 • CYL110 - Physical Chemistry: Concepts and Applications [4 cr. (3-1-0)] • Semester I 2012-13, M, Th (9:30- 11), Tue-Thurs 2:003:00 (MS418) • Instructors: Dr. Shashank Deep (SD) and Dr. Pramit K Chowdhury(PKC) • Course Coordinator: Prof. Siddharth Pandey (PKC) • Office: MS731 (SD) • Phone: 6596 (SD) • E-mail: [email protected] (SD) CYL 110 Grading • Grading based on: • • • • • Minor Tests I Minor Tests II 2 Quizzes Major Test Passing grade is 30%. 20% 20% 20% 40% Recommended books • Books and Reference Material: • (i) Physical Chemistry by Atkins and de Paula • (ii) The Elements of Physical Chemistry by Atkins • (iii) Physical Chemistry by Silbey and Alberty • (iv) Physical Chemistry by Levine • (vi) Physical Chemistry: A Molecular Approach by McQuarrie and Simon • (vii) Physical Chemistry by Laidler, Meiser, and Sanctuary Course contents (chemical Thermodynamics) • Zeroth law of themodynamics, Ideal and real gases, van der Waals and virial equations of state, Critical point. • First law of thermodynamics and internal energy, Exact and inexact differentials, Isothermal and adiabatic processes, Enthalpy, Joule-Thompson expansion, Heat Capacities. • Second law of thermodynamics and entropy, entropy changes in reversible and irreversible processes. Course contents (Chemical Thermodynamics) • Combined first and second law, thermodynamic potentials, free energy and work, effect of temperature and pressure on free energy. • Chemical potential, equilibrium and free energy, phase equilibria: phase rule, phase equilibrium of onecomponent system, clapeyron equation, clausius clapeyron equation, colligative properties. • Gaseous equilibrium, Le Chatelier’s principle, van’t Hoff equation. CYL110 Tutorials • http://web.iitd.ac.in/~sdeep • Menu • Tutorial I-V Courses CYL110 Thermodynamics • Effects of gravitational field, centrifugal field and surface area on the properties of the system. • Phase transitions like graphite to diamond conversion, helium normal to superfluid transition, conductor to semiconductor transition, change of boiling point of a liquid with pressure or addition of solute. • Biochemistry-Enzymes and protein stability, DNA stability, metabolic processes leading to mechanical work performed by a living organism, design of drugs. Thermo---------• Industrial Chemistry-Different chemical processes like synthesis of ammonia from nitrogen and hydrogen. • Thermodynamics of complexation with macrocyclic ligands- A system of interest in Inorganic chemistry and chemical separation. • Geological problems- solubility of calcite, energetics of ternary oxides of minerological significance. A thermodynamic system is that portion of the Universe that we have selected for investigation •The surroundings are everything outside the system. The boundary separates the system from the surroundings The state of a system is defined as the complete set of all its properties which can change during various specified processes. When a system is at equilibrium, its state is defined entirely by the state variables, and not by the history of the system. The properties of the system can be described by an equation of state which specifies the relationship between these variables. System-------------------------- GAS Variables---------------------- n, P,V,T Equation of State -------- PV=nRT A perfect gas is defined as “A gas where intermolecular forces are negligible”. Temperature • Zeroth law of thermodynamics. • Do we need definition of temperature ? • On a cold winters day a metal railing feels much colder than a wooden fence post, but they are both at the same temperature. • The zeroth law states “If two systems are separately in thermal equilibrium with a third, then they must also be in thermal equilibrium with each other.” Bulk Variables • V, the Volume the sample occupies (m3) m3 = 106 cc = 1000 L • p, the Pressure of the sample (atm) 1 atm = 101.325 kPa = 1.01 bar = 760 Torr • T, the Temperature of the sample (K) • n, Composition - moles Ideal Gas Model • Molecules may be treated as point masses relative to the volume of the system. • Molecular collisions are elastic, i.e. kinetic energy is conserved. • Intermolecular forces of attraction and repulsion have negligible effect on the molecular motion. Compressibility • The compressibility of a gas is defined by pVm Z RT • If the gas behaves ideally, then Z=1 at all pressures and temperatures. • Z >1 molecules occupy more volume than IG (e.g. H2): repulsive forces • Z < 1 molecules occupy less volume than IG (e.g. CO2): attractive forces Critical constants of some gases • The very low critical pressure and temperature of helium, reflecting the very small intermolecular attractions of this atom. • Tc of the noble gas elements increases with atomic number. • Hydrogen gas cannot be liquified above 33 K; this poses a major difficulty in the use of hydrogen as an automotive fuel; storage as a high-pressure gas requires heavy steel containers which add greatly to its effective weight-per-joule of energy storage. • The properties of carbon dioxide (particularly its use as a supercritical fluid). • The high Tc of H2O is another manifestation of its "anomalous" properties relating to hydrogen-bonding. Calculation of critical point a point on a curve at which the tangent crosses the curve itself. a point on a curve at which the curvature changes sign. A point on a curve at which the second derivative changes sign. a point (x,y) on a function, f(x), at which the first derivative, f'(x), is at an extremum, i.e. a minimum or maximum. Super critical fluid (Gases above their TC and Pc) It possesses the flow properties of a gas and the solvent properties of a liquid. Supercritical Fluid is an example of gas which can dissolve solids. The density of a supercritical fluid can be changed over a wide range by adjusting the pressure; this, in turn, changes its solubility, which can thus be optimized for a particular application. Soluble components are extracted from a substrate by a high pressure gas, and the extracted components that have been dissolved in the gas are precipitated from the gas when the pressure is reduced. Major drawback in using liquids as a solvent is the presence of residual solvents in the product. Super critical fluids are used for decaffeinating coffee and tea and extracting flavors, aroma and essential oils from hops, spices, flowers and herbs. Virial equation of state It is based on statistical mechanical theory, where each power level indicates a higher level of interaction. The virial equation does not tend to be very good at high densities (low T, high P). Boyle’s temperature Z 0 P T Z 0 1 V m B0 as P0 as V van der Waals Equation p a / V V 2 m m b nRT Vm,eff Vm b repulsion peff p a / V attraction 2 m van der Waals constants a (dm6 atm mole-1) b (dm mole-1) He 0.034 0.0237 Ar 1.345 0.0322 N2 1.390 0.0391 O2 1.360 0.0318 CO2 3.592 0.0427