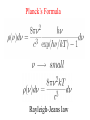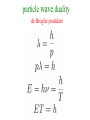* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Quantum Mechanics: Introduction
Coherent states wikipedia , lookup
Quantum chaos wikipedia , lookup
Standard Model wikipedia , lookup
Path integral formulation wikipedia , lookup
Future Circular Collider wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Monte Carlo methods for electron transport wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
ATLAS experiment wikipedia , lookup
Identical particles wikipedia , lookup
Quantum tunnelling wikipedia , lookup
Renormalization wikipedia , lookup
Canonical quantization wikipedia , lookup
Wave function wikipedia , lookup
Elementary particle wikipedia , lookup
Relational approach to quantum physics wikipedia , lookup
Photoelectric effect wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Uncertainty principle wikipedia , lookup
Photon polarization wikipedia , lookup
Wave packet wikipedia , lookup
Old quantum theory wikipedia , lookup
Electron scattering wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Birthday of Quantum Physics on 14th December, 1900 E h Planck introduces a new fundamental constant Max Karl Ernst Ludwig Planck 1858-1947 h to explain black-body radiation Blackbody Radiation ~ Energy density in the window υ and υ +d υ ρ(υ) 3 J/m -Hz hυ = kT h :Planck’s constant = 6.63 x 10 -34 J-s k=:Boltzmann’s const. = 1.38 x 10 = R/N -23 T=1800 K T=1400 K T=1000 K frequency υ J/K Classical concept Two distinct categories : 1. Material body (particle) Newton’s laws of motion Position and velocity (momentum) are precisely measurable Spread over the space, amplitude gives 2. Electromagnetic field (wave) energy/intensity, frequency is nothing Maxwell’s equation but time periodicity of oscillator Additionally Laws of thermodynamics E = kT Fundamental constants : 1. velocity of light c 2. Avogadro Number N 3. Boltzman constant k 4. Unit of charge e E = mc2 Velocity << c : non-relativistic Velocity comparable to c : relativistic Classical concepts never allow to think that 1. Wave may also behave like particle. (Planck’s hypothesis) 2. Particle may behave like wave. (de Broglie hypothesis) 3. Position and momentum of a particle cannot be measured accurately simultaneously. (Heisenberg uncertainty principle) 4. Energy of wave is related with frequency and quantised. E nh These new concepts is basically quantum concepts Rayleigh-Jeans law Classical theory T=1800 K ρ(υ) T=1800 K T=1400 K T=1000 K frequency υ ρ(υ) T=1800 K υ dependence 2 frequency υ Planck’s Formula Rayleigh-Jeans law Planck’s postulate Any physical entity with one degree of freedom and whose ``co-ordinate” is oscillating sinusoidally with frequency can possess only total energies E as integral multiple of h = Planck’s constant 4 Classical 2 E=0 particle wave duality de Broglie postulate waves behaving as particles Experiments 1. Photoelectric effect (1902) 2. Compton effect (1922) 3. Pair Production particles behaving as waves Experiments 1. Electron diffraction Davisson –Germer (USA) and Thompson (UK) (1927) 2. Electron microscope