* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Building Blocks of Life
Interactome wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Western blot wikipedia , lookup
Genetic code wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photosynthesis wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Signal transduction wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
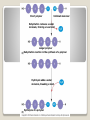
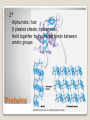
Building Blocks of Life An Introduction Carbon is unparalleled in its ability to form large, complex, and diverse molecules • Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds • Carbon—The Backbone of Biological Molecules Electron configuration determines the kinds and number of bonds an atom will form with other atoms With four valence electrons, carbon can form four covalent bonds with a variety of atoms ◦ makes large, complex molecules possible Carbon atoms can form diverse molecules by bonding to four other atoms The valences of carbon and its most frequent partners (hydrogen, oxygen, and nitrogen) are the “building code” that governs the architecture of living molecules Within cells, small organic molecules are joined together to form larger molecules Macromolecules are large molecules composed of thousands of covalently connected atoms Macromolecules Monomers build polymers linked together by covalent bonds • Three of the four classes of life’s organic molecules are polymers: • ◦ ◦ ◦ ◦ Carbohydrates Proteins Nucleic acids Lipids polymers built from monomers Monomers form larger molecules by condensation reactions called dehydration reactions Polymers are disassembled to monomers by hydrolysis, a reaction that is essentially the reverse of the dehydration reaction The Synthesis and Breakdown of Polymers Short polymer Unlinked monomer Dehydration removes a water molecule, forming a new bond Longer polymer Dehydration reaction in the synthesis of a polymer Hydrolysis adds a water molecule, breaking a bond Hydrolysis of a polymer Sugars and sugar polymers Monosaccharides ◦ Simple sugars ◦ glucose Carbonyl group Hydroxyl group Carbohydrates Disaccharides ◦ 2 or more monosaccharides joined by glycosidic linkage, covalent bond by dehydration reaction ◦ Glucose + fructose sucrose Carbohydrates Storage ◦ Plant starch ◦ Stored energy can be broken down by hydrolysis into glucose ◦ Animal polysaccharide Glycogen ◦ Stored in liver and muscles ◦ Used for short term energy Carbohydrates Structure ◦ Cellulose: cell walls Requires an enzyme for animals to break it down ◦ Chitin: exoskeleton of arthropods and fungi Carbohydrates Fats, oils, waxes ◦ Mix poorly with water ◦ Fats Large molecules of glycerol and fatty acid chains connected by dehydration Lipids Cell Membranes ◦ Phospholipid bi-layer Lipids Polymer of amino acids called polypeptides Functions ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Enzymes Storage of amino acids Hormones Motor Defense Transport Receptors for chemical stimuli structure Proteins Amino acids ◦ 20 amino acids from 1000’s of proteins ◦ Side chains “R” determines the properties Hydrophillic:polar Hydrophobic: non polar Hydrophillic: electric charge Proteins Structure ◦ 1° Linear chain Proteins 2° ◦ Alpha helix: hair ◦ β pleated sheets: spider web ◦ Held together by hydrogen bonds between amino groups Proteins 3° ◦ Interactions between side chains “R” Hydrogen bonds Ionic bonds Disulfide bonds Van der Waals Proteins 4° ◦ Aggregation of polypeptide subunits Collagen hemoglobin Proteins Denaturation ◦ Weak chemical bonds and interactions can be destroyed Heat pH Proteins Polymer of nucleotides ◦ DNA and RNA Nucleic Acids