* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 12.26MB - Stanford University
Composition of Mars wikipedia , lookup
Geochemistry wikipedia , lookup
Crystallization wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Thermal spraying wikipedia , lookup
Nanoparticle wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Brownian motion wikipedia , lookup
Colloidal crystal wikipedia , lookup
Metalloprotein wikipedia , lookup
Freeze-casting wikipedia , lookup
Stöber process wikipedia , lookup
Acid–base reaction wikipedia , lookup
Liquid-feed flame spray pyrolysis wikipedia , lookup
Atomic theory wikipedia , lookup
Elementary particle wikipedia , lookup
Aerosol mass spectrometry wikipedia , lookup
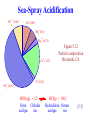
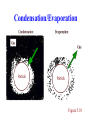
Presentation Slides for Atmospheric Pollution: History, Science, and Regulation Chapter 5: Aerosol Particles in Smog and the Global Environment By Mark Z. Jacobson Cambridge University Press, 399 pp. (2002) Last update: March 23, 2005 The photographs shown here appear in the textbook and are provided to facilitate their display during course instruction. Permissions for publication of photographs must be requested from individual copyright holders. The source of each photograph is given below the figure and in the back of the textbook. Particle Characteristics Mode Diameter (mm) Gas molecules 0.0005 Aerosol particles Small < 0.2 Medium 0.2-2 Large >2 Hydrometeor particles Fog drops 10-20 Cloud drops 10-200 Drizzle 200-1000 Raindrops 1000-8000 Number (#/cm3) 2.45x1019 103-106 1-104 <1 - 10 1-1000 1-1000 0.01-1 0.001-0.01 Particle Size Distribution Variation of particle concentration (i.e., number, surface area, volume, or mass of particles per unit volume of air) with size. Size distribution usually divided into modes Mode Diameter Range (mm) Nucleation < 0.1 Accumulation 0.1 - 2 Submode 1 ~ 0.2 Submode 2 ~ 0.5 - 0.7 Coarse >2 Lognormal Distribution 10 ) / d log D (m m) 10 2 1 10 0 -1 -3 dv m (m 3 3 cm-3 10 10-2 -3 10 0.001 dv (mm cm ) / d log 10 10D (mm) dv -3)-3 / 3 cm dv 3m (m (mm cm ) / d log m) d log1010DD(m(mm) Describes an individual mode of a size distribution D1 D2 0.01 0.1 Particle diameter (D, mm) 1 10 2 10 1 10 0 10 -1 10-2 10 -3 0 0.05 0.1 0.15 Particle diameter (D, mm) Figure 5.1 Ambient Size Distribution 10 2 10 1 10 0 10-1 0.01 2 n 250 x=a ( mm cm ) -3 200 x=v ( mm cm ) 3 -3 150 10 dn (particles cm ) / d log -3 -3 4 10 10 3 300 100 50 0 0.1 1 Particle diameter (D, mm) dx / d log10DD (mm) (m m) 10 6 10 5 dx / d log D (10 dn (particles cm ) / d10log D (mm) m m) Figure 5.2, Claremont, California, Aug., 1987 Mode Diameter Range (mm) Nucleation < 0.1 Accumulation 0.1 - 2 Submode 1 ~ 0.2 Submode 2 ~ 0.5 - 0.7 Coarse >2 10 Sources of New Particles Emission Sea spray Soil dust Volcanic Biomass burning Fossil-fuel combustion Industrial Miscellaneous Homogeneous nucleation Homomolecular Binary Ternary Sea Spray Emission Form when winds and waves force air bubbles to burst at sea surface Contain composition of sea water Spume drops Drops larger than sea spray form when winds tear off wave crests. Sea-spray acidification Reduction in chloride in sea spray drops as sulfuric acid or nitric acid enter the drops. Dehydration Water loss when drop evaporates in low relative-humidity air Constituents of Sea Water Constituent Water Sodium Chlorine Magnesium Sulfur Calcium Potassium Carbon Mass percent 96.78 1.05 1.88 0.125 0.0876 0.0398 0.0386 0.0027 Table 5.3 Sea-Spray Acidification SO 4 2- (0.66) + Na (0.66) + NH (0.5) 4 2+ M g (0.23) 2+ Ca (1.47) Figure 5.12 Particle composition Riverside, CA - - NO (4.06) Cl (0.12) 3 HNO3(g) + ClNitric Chloride acid gas ion HCl(g) + NO3Hydrochloric Nitrate acid gas ion (5.5) Soil Dust Emission Soil Natural, unconsolidated mineral and organic matter lying above bedrock on the surface of the Earth. Originates from the breakdown of rocks and decay of dead plants and animals. Rocks Sedimentary: Cover 75 percent of Earth’s surface and form by deposition and cementation of carbonates, sulfates, chlorides, shell fragments. Example: chalk. Igneous: Form by cooling of magma. Example: Granite. Metamorphic: Form by structure transformation of existing rocks due to high temperatures and pressures. Example: marble. Breakdown of Rocks to Soil Physical weathering Disintegration of rocks and minerals by processes not involving chemical reactions. Examples: when stress applied to a rock. Stresses arise due to high pressure under soil or when rocks freeze/thaw or when saline solutions enter cracks and cause disintegration/fracture. Chemical weathering Disintegration of rocks and minerals by chemical reaction. Example: Dissolution of gypsum in water: CaSO 4-2H2O(s) Calcium sulfate dihy drate (gypsum) Ca2+ + SO 4 2- + 2H2 O(aq) (5.1) Calcium Sulfate Liquid ion ion water Types of Minerals in Soil Dust Quartz - SiO2(s) - clear, colorless, resistant to chemical weathering Feldspars - 50 percent of rocks on Earth’s surface Potassium feldspar - KAlSi3O3(s) Plagioclase feldspar - NaAlSi3O3-CaAl2Si2O8(s) Hematite - Fe2O3(s) - reddish brown Calcite - CaCO3(s) - found in limestone Dolomite - CaMg(CO3)2(s) Gypsum - CaSO4-2H2O(s)- colorless to white Clays - soft, compact, odorous minerals resulting from weathering Kaolinite - Al4Si4O10(OH)8(s) Illite Smectite Vermiculite Chlorite Organic matter - plant litter or animal tissue broken by bacteria Dust Storm From Africa (Feb. 28, 2000) SeaWiFS Project, NASA/Goddard Space Flight Center and ORBIMAGE Time For Particles to Fall 1 km Diam. (mm) 0.02 0.1 1.0 10 100 1000 5000 Time to Fall 1 km 228 y 36 y 328 d 3.6 d 1.1 h 4m 1.8 m Only particles smaller than 10 mm reach the global atmosphere Table 5.4 Volcanic Emission Over 500 volcanos currently active Magma contains 1-4 percent gas by mass. Water vapor makes up 50-80 percent of gas mass Some other constituents: CO2(g) SO2(g) OCS(g) N2(g) CO(g) H2(g) S2(g) HCl(g) Cl2(g) F2(g) Particles Most abundant are silicate minerals. Range in size from <0.1 to 100 mm Mount St. Helens (Fall, 1982) Peter Frenzen, available from Mount Saint Helens National Volcanic Monument Photo Gallery Biomass-Burning Emission Burning of evergreen forests, deciduous forests, woodlands, grasslands, agricultural land either to clear land, stimulate grass growth, manage forest growth, or satisfy a ritual. Gas constituents CO(g) CO2(g) CH4(g) NOx(g) ROGs SO2(g) Particle constituents Ash, plant fibers, soil dust, organic matter, soot (black carbon plus organic matter) Composition of soot High temperatures: Higher ratio of BC:OM in soot. Biomass Burning in South Africa (September 7, 2000) NASA/GSFC/JPL, MISR and Air MISR Teams Ash, Combusted Plant Fiber, Elongated Ash, Soil Dust Reid and Hobbs (1998) Prescribed Burn in Big Horn National Forest, Wyoming (1981) Fig. 5.7. U.S. Forest Service, available from National Renewable Energy Lab. Fossil Fuels Coal Combustible brown-to-black carbonaceous sedimentary rock formed by compaction of partially decomposed plant material. Stages of coal metamorphosis Peat (unconsolidated, brown-black) Peat coal (consolidated, brown-black) Lignite coal (brown-black) Bituminous (soft) coal (dark brown to black) Anthracite (hard) coal (black) Oil (petroleum) Natural greasy, viscous, combustible hydrocarbon liquid that forms from geological-scale decomposition of plants and animals. Fossil Fuels Natural gas Colorless, flammable gas, made primarily of methane, often found near petroleum deposits. Kerosene Combustible, oily, water-white liquid with strong odor distilled from petroleum Diesel Combustible liquid distilled from petroleum after kerosene. Fossil-Fuel Combustion Emission Gases NOx(g), ROGs(g), CO(g), CO2(g), CH4(g), SO2(g) Particles Soot (BC+OM), OM alone, SO42-, metals, fly ash Fly ash Contains O, Si, Al, Fe, Ca, Mg. Industrial Emission Source Smelters Oil-fired power plants Coal-fired power plants Metals in Fly Ash Fe, Cd, Zn V, Ni, Fe Fe, Zn, Pb, V, Mn, Cr, Cu, Ni, As, Co, Cd, Sb, Hg Municipal waste incineration Zn, Fe, Hg, Pb, Sn, As, Cd, Co, Cu, Mn, Ni, Sb Steel-mill furnaces Fe, Zn, Cr, Cu, Mn, Ni, Pb Table 5.5 Miscellaneous Particle Sources Tire-rubber particles Pollen Spores Bacteria Viruses Plant debris Meteoric debris Processes Affecting Particle Evolution Homogeneous nucleation Emission Coagulation Growth Condensation/evaporation Deposition/sublimation Dissolution, dissociation, hydration Solid precipitation Dry deposition Rainout Washout Coagulation Occurs when two particles collide and stick together (coalesce). Reduces number concentration but conserves volume concentration Classic coagulation mechanisms Brownian motion Convective Brownian diffusion enhancement Gravitational collection Turbulent inertial motion Turbulent shear Additional mechanisms Diffusiophoresis Thermophoresis Electric charge van der Waals forces Fractal geometry effects 80 4 10 10 3 10 2 10 60 After 1 day 40 1 0 10 10 -1 0.01 Initial After 1 day 20 0 0.1 1 Particle diameter (D, mm) 10 (mm) m m) m) dn (particles cm-3) / d log10Dm(mm) Initial log10D -3 100 ( m cm dv m 10 10 5 dv (mm3 3cm-3)-3/ d 6 ) / d log10 D ( dn (partic. cm ) / d log10 D ( Effect of Brownian Coagulation Figure 5.9 Condensation/Evaporation Figure 5.10 Condensing Gases Condensation occurs primarily on accumulation mode since it contains the largest surface area concentration of all modes. Water vapor Condenses on accumulation and coarse-mode particles to form cloud drops Sulfuric acid Condensation onto accumulation mode affects visibility High-molecular weight organic gases Products of toluene, xylene, alkylbenzene, alkane, alkene, biogenic hydrocarbon oxidation condense onto accumulation mode primarily. 80 60 40 Over liquid water 20 0 -20 -10 0 10 20 30 40 50 o Temperature ( C) 8 Vapor pressure (mb) 100 Vapor pressure (mb) 120 Vapor pressure (mb) Vapor pressure (mb) Saturation Vapor Pressure of Water Over Liquid Water/Ice Surfaces 7 6 5 4 3 Over liquid water 2 1 0 -50 Over ice -40 -30 -20 -10 o Temperature ( C) 0 Figure 5.11 Dissolution Dissolution Process by which a gas diffuses to and dissolves in a liquid on a particle surface. Solvent A liquid in which a gas dissolves Solute Gas, liquid, or solid that dissolves in a solvent Solution One or more solutes plus the solvent. Solubility Maximum gas that can dissolve in a quantity of solvent Common dissolving gases HCl(g), HNO3(g), NH3 (g), SO2(g) Dissociation Dissociation Breakdown of dissolved molecules into ions. Cations Positively-charged ions (e.g., H+, Na+, K+, Ca2+) Anions Negatively-charged ions (e.g., OH-, Cl-, NO3-, HSO4-, SO42-) Electrolyte Substance that undergoes partial or complete dissociation Acidity Measure of the concentration of hydrogen ions (H+) in solution pH = -log10[H+] [H+] = molarity (M, moles of H+ per liter of solution) Higher [H+] --> lower pH --> more acidic solution (5.2) Acidity In dilute water, the only source of H+ is H2O(aq) Liquid water H+ + OHHydrogen Hydroxide ion ion [H+][OH-] = 10-14 M2 --> [H+]=[OH-]=10-7 M --> pH = -log10[10-7M] = 7 (5.3) Acid/Base Acid Substance that, when added to a solution, dissociates, increasing [H+], decreasing pH Strong acid: Substances that dissociate readily (e.g., H2SO4, HCl, HNO3) Weak acids: Substances that dissociate less readily (e.g., H2CO3) Base (alkalis) Substances that, when added to a solution, reduce [H+], increasing pH. (e.g., NH3, Ca(OH)2) pH Scale Figure 10.3 Dissolution/Dissociation Addition of acid to solution increases [H+], decreasing pH Hydrochloric acid HCl(g) HCl(aq) H+ + ClHydrogen Chloride Hydrochloric Dissolved ion ion acid gas hydrochloric acid Nitric acid HNO3(g) Nitric acid gas HNO3(aq) Dissolved nitric acid H+ + NO3Hydrogen Nitrate ion ion (5.4) - (5.6) Soil-Particle Acidification Addition of acid to calcite-containing soil dust removes carbonate ion CaCO 3(s) + 2HNO3(g) Calcium carbonate Nitric acid gas Ca2+ + 2NO3- + CO2(g) + H 2O(aq) Calcium Nitrate Carbon ion ion dioxide gas Liquid water (5.7) Hydration Bonding of liquid water to solute (anions, cations, or undissociated molecules). The higher the relative humidity and greater the quantity of solute, the greater the liquid water uptake due to hydration. Hydration responsible for swelling of particles sufficiently to cause a haze when the relative humidity is below 100 percent. (5.3) Sulfuric Acid Condensation/Dissociation H2SO4(g) Sulfuric acid gas H2SO4(aq) Dissolved sulfuric acid H+ + HSO4Hydrogen Bisulfate ion ion 2H+ + SO 42Hydrogen Sulfate ion ion Condensation occurs primarily on accumulation mode (5.8) Ammonia Dissolution/Dissociation Stationary-source fuel burning (2) Other stationary sources (3) Mobile combustion sources (2) Fertilizers (5) Sewage treatment plants (8) Livestock (52) Soils (14) Figure 5.13 Ammonia gas emissions Los Angeles Humans/domestic animals (14) NH3(g) Ammonia gas NH3(aq) Dissolved ammonia NH3(aq) + H+ Dissolved Hydrogen ammonia ion NH4+ Ammonium ion (5.9) - (5.10) Na 10 + NH 4 8 2+ Long Beach, California August 29, 1987 0500-0830 PST Mg 2+ Ca 6 4 Cl - 3 SO 2 - NO 2- 4 0 0.075 0.14 0.27 0.52 1.04 2.15 4.35 Stage 50-percent cutoff diameter mm) ( 8.2 -3 + ) m-3) Stage concentrationm g m(mg 12 Stage concentration ( -3 m g m(mg ) m-3) Stage concentration Stage concentration ( Aerosol Particle Composition vs. Size 40 35 + Na + NH 4 30 2+ Mg 25 Riverside, California August 29, 1987 0500-0830 PST 2+ Ca 20 Cl 15 NO 10 SO - 3 2- 4 5 0 0.075 0.14 0.27 0.52 1.04 2.15 4.35 Stage 50-percent cutoff diameter mm) ( Figure 5.14 8.2 Solid Precipitation When the relative humidity decreases, ions in solutions may combine (crystallize) to form solids (solid precipitation). Solids may also form by chemical reaction on the surface of particles. Common solids Ammonium nitrate - NH4NO3(s) Ammonium sulfate - (NH4)2SO4(s) Gypsum - CaSO4-2H2O(s) Composition, Sources of Particles Nucleation Mode Nucleation H2O(aq), SO42-, NH4+ Accumulation Mode Fossil-fuel emission BC, OM, SO42Fe, Zn Fossil-fuel emission Biomass-burning BC, OM, SO42-, BC, OM, SO42-, ClFe, Zn Fe, Mn, Zn, Pb, V, Cd, Cu, Co, Sb, As, Ni, Cr Coarse Mode Sea-spray emission H2O(aq), Na+, Ca2+, Mg2+, K+, Cl-, SO42-, Br-, OM Soil-dust emission Si, Al, Fe, Ti, P, Mn, Co, Ni, Cr, Na+, Ca2+ Mg2+, K+, SO42-, ClCO32-, OM Table 5.6 Composition, Sources of Particles Nucleation Mode Biomass burning BC, OM, SO42-,ClMn, Zn, Pb, V, Cd, Cu, Co, Sb,As, Ni, Cr Accumulation Mode Industrial emission BC, OM, Fe, Al, S P, Mn, Zn, Pb, Ba Sr, V, Cd, Cu, Co Hg, Sb, As, Se, Ni, H2O(aq), NH4+, Na+, Ca2+, K+, SO42-, NO3-, Cl-, CO32- Coarse Mode Biomass-burning ash, industrial fly ash, tire-particle emission Cond./dissolution H2O(aq), SO42-, NH4+, OM Cond./dissolution H2O(aq), SO42-, NH4+, OM Cond./dissolution H2O(aq), NO3- Coagulation Coagulation Particle Mixing State External mixture Particle composition is the same as that when the particle was emitted. Internal mixture Particle composition differs from that when it was emitted due to condensation, dissolution, coagulation, and other physical processes affecting composition. Internally- and Externally-Mixed Soot Particles Soot inclusion Pósfai et al. (1999) Strawa et al. (1999) Particle Health Effects Hazardous compounds in particles Benzene, PAHs, metals, sulfur compounds Metals Lung injury, bronchioconstriction, increased infection PM10 Asthma, chronic obstructive pulmonary disease, increased mortality, higher hospitalization and health-care visits for respiratory and cardiovascular disease. May be no low threshold for health-related problems due to PM10. PM2.5 More respiratory illness and premature death than larger particles. Long-term city exposure may reduce life by two years. Total air pollution mortality (due mostly due to particles) 2.7 million deaths/yr worldwide from air pollution; 1.8 million in rural areas, mostly due to indoor burning of biomass, coal.