* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pollution of Lakes and Rivers Chapter 15
Climate change feedback wikipedia , lookup
Global warming wikipedia , lookup
Fred Singer wikipedia , lookup
IPCC Fourth Assessment Report wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Clean Air Act (United States) wikipedia , lookup
Politics of global warming wikipedia , lookup
Climate change, industry and society wikipedia , lookup
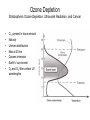
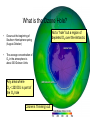
Pollution of Lakes and Rivers Chapter 15: Ozone depletion, acid rain, and climatic warming: the problems of multiple stressors Copyright © 2008 by DBS Contents • • • • • • • The challenges of multiple stressors Stratospheric ozone depletion, ultraviolet radiation, and cancer Refrigerators, air conditioners, aerosol cans, and the ozone layer Reconstructing past UV penetration in lakes Paleo-optics: tracking long-term changes in the penetration of ultraviolet radiation in lakes Biological responses to enhanced UV-B exposure: evidence from fossil pigments Multiple stressors: new combinations of old problems Ozone Depletion The Challenges of Multiple Stressors • ‘several environmental problems culminating into a larger, potentially more serious calamity’ • 3 human-related alterations of the atmosphere: – Climate change – Acid deposition – Ozone depletion • Negative impacts are significantly amplified when they occur simultaneously – multiple stressors Cartoon Allotropes of Oxygen Allotropes of Oxygen Chapman Theory Above stratosphere oxygen absorbs UV-C and exists as O atoms O2 + hν → O + O ΔH = 495 kJ/mol (<241 nm) (1) + O3 Oxygen atom could react with oxygen molecule to form O3 O + O2 + M → O 3 ΔH = -100 kJ/mol (2) O3 formed could react with O atoms or absorb solar radiation O3 + hν → O2 + O O + O3 → 2O2 - (<320 nm) ΔH = -390 kJ/mol (3) - O3 (4) A third molecule ‘M’ (N2 or H2O) facilitates as a heat energy carrier (is not required when there is more than one molecule produced) Enthalpies show a great deal of heat is generated Because of the temperature inversion vertical mixing of air is slow in the stratosphere Chapman Theory Photostationary state is established O3 + hν ↔ O2 + O Net effect: one form of ‘odd oxygen’ is converted into another (O3 to O) • At night back reaction dominates • At day forward reaction dominates (hν) JO3 Ozone Depletion Stratospheric Ozone Depletion, Ultraviolet Radiation, and Cancer • • • • • • • O3 present in trace amounts Natural Uneven distribution Max at 25 km Causes inversion Earth’s ‘sun-screen’ O2 and O3 filter certain UV wavelengths Solar Flux Phytoplankton UV-B damage Melanoma O2 + O3 as Natural UV Filter absorbs transmits Absorption of UV-C and UV-B by O3 What is a Dobson Unit? • • • 1 DU is the number of molecules of O3 required to create a layer of O3 0.01 mm (0.001 cm) thick at 0 °C and 1 atm A column of air with an O3 concentration of 1 DU would contain about 2.69 x 1016 O3 molecules cm-2 at the base of the column Over the Earth’s surface, the O3 layer’s average thickness is about 300 DU (3 mm layer) if brought to sea level O3 “Hole” [O3] ~100 DU What is the Ozone Hole? • Occurs at the beginning of Southern Hemisphere spring (August-October) • The average concentration of O3 in the atmosphere is about 300 Dobson Units Any area where O3 < 220 DU is part of the O3 hole Ozone is ‘thinning’ out Not a “hole” but a region of depleted O3 over the Antarctic Mid-lattitudes • • Arosa, Switzerland Continuous record back to 1931 • Slow, steady decline, of about 3% per decade during the past twenty years • Enhanced by volcanic eruptions (Mt. Pinatubo) Kerr, 2002 Natural change or related to human activities? Summary The Big Surprise of 1985 • Farman et al. revealed a dramatic and unpredicted decline in stratospheric O3 in a surprising location – Antarctica – Showed dramatic decline in springtime O3 starting in 1970’s 30% by 1985 70% by 2000 Chemical explanation? Min O3 at Antarctic in Spring (Sep-Nov) Physical explanation? Ozone Destruction • In september destruction occurs ~2 % per day • Most O3 is wiped out at 1520 km altitude Could not be explained by natural cycles since under low light conditions [O] is too low (UV-C required to produce O does not penetrate past 20 km) Ozone Depletion Refrigerators, Air Conditioners, Aerosol Cans, and the Ozone Layer • • • Known since 1970’s that human produced chlorine and bromine gases destroy ozone CFC’s inert in troposphere but sink for O3 in stratosphere Uses: air conditioning, refrigerators, aerosol cans, plastic foams etc. CFCl3 + hν → CFCl2 + •Cl Ozone Depletion Refrigerators, Air Conditioners, Aerosol Cans, and the Ozone Layer Catalyst X (chlorine or bromine atom) cleaves an oxygen (O) atom from an ozone (O3) molecule, forming OX and O2 Oxygen atom (O) can then remove the oxygen atom attached to the catalyst, releasing the catalyst •X + O3 → •XO + O2 •XO + O → •X + O2 O 3 + O → O 2 + O2 Ozone Depletion Refrigerators, Air Conditioners, Aerosol Cans, and the Ozone Layer • Increased UV-B penetration should be cause for concern Humic and fulvic fractions of DOC absorbs strongly (Vincent and Roy, 1993) and act as natural sun-screen in lakes With less DOC UV-B penetration is greater (Vincent and Pienitz, 1996) As DOC concentrations increase, the depth of UV-B penetration decreases Schindler et al. (1996) • • Ozone Depletion Refrigerators, Air Conditioners, Aerosol Cans, and the Ozone Layer Triple whammy • Situation is made worse due to combined effects of our multiple stressors – acid precipitation (Yan et al, 1996) and – warming (Schindler et al, 1996) • 3 stressors act in concert to increase UV-B exposure of aquatic organisms Ozone Depletion Refrigerators, Air Conditioners, Aerosol Cans, and the Ozone Layer The top panel shows in their “natural states”, with DOC being delivered to the lake from the catchment Acidity - The middle panel shows how DOC in the lake is reduced with acidic precipitation: (i) increases photodegradation of DOC, (ii) precipitates DOC from water column – clearer lakes Warming - The lower panel shows how the supply of DOC to the lake is further diminished under drought conditions, as less DOC is delivered to the lake Ozone Depletion Reconstructing Past UV Penetration in Lakes • No direct information, rely on indirect methods: 1. Infer past lakewater DOC (Pienitz et al, 1999), reconstruct past underwater radiation climate (paleo-optics) and relate this to vegetation change (Pienitz and Vincent, 2000) or acidic deposition (Dixit et al, 2001) 2. Track past UV-B penetration by studying biotic response – pigment changes in blue-green algae (Leavitt et al, 1997) Ozone Depletion Paleo-optics: Tracking Long-term Changes in the Penetration of UV Radiation • • Boreal forest lakes have high DOC, biota relatively immune from damage Diatom transfer functions used to reconstruct past DOC • Data has been gathered for Arctic tree line regions where it is used to reconstruct position of the coniferous tree line (Pienitz et al, 1997) Canadian Sub-Arctic, Queens Lake… • Ozone Depletion Pienitz and Vincent, 2000 Paleo-optics: Tracking Long-term Changes in the Penetration of UV Radiation Using a diatom DOC transfer function researchers inferred a warming period between 5000 – 3000 yrs BP Ozone Depletion Biological Responses to Enhanced UV-B Exposure: Evidence from Fossil Pigments • Biological response may be directly related to UV • Leavitt et al (1997,1999) has shown use of fossil pigments to track UV (i) alpine lakes subjected to droughts (ii) artificially acidified lake in Ontario • Alpine study – pigments increase c. 1850 – 1900 Suggests higher UV penetration Tree ring data suggests cool weather and droughts dec. DOC • • Biological Responses… • Fossil pigments increased in post acidification sediments of Lake 302S (Exp. Lakes Area) • • Lake was artifically acidified in 1980 Lead to decline in lakewater DOC Concentrations of UV-absorbing fossil pigment in the sediments of Lake 302S, NW Ontario. This lake was artificially acidified in 1980, resulting in a decrease in DOC and a corresponding increase in UV-B penetration. Leavitt et al (1999) Ozone Depletion Ozone Depletion Multiple stressors: New Combinations of Old Problems • A Ozone Depletion Summary • • • • • Ozone depletion, acid rain and climate change are often discussed in isolation Cumulative effects may result in more severe damage Decreases in the DOC due to acidification and rising temperatures may have severely affected a lakes ability to filter out harmful UV-B radiation Paleolimnological methods used to track changes in lake DOC include diatom transfer functions and fossil pigments As new problems are constantly being identified, dealing with multiple stressors is possibly the greatest challenge facing environmental scientists for the foreseeable future. References • • • • • • • • • • Battarbee (2000) Blokker et al (2005) Dixit et al (2001) Gorham (1996) Hengeveld (1991) Hodgson et al (2005) Leavitt, P.R., Vinebrooke, R., Donald, D.B., Smol, J.P. and Schindler, D.W. (1997) Past ultraviolet radiation environments revealed using fossil pigments in lakes. Nature, Vol. 388, pp. 457-459. Leavitt et al (1999) Leavitt et al, (2003) Laurion et al (1997) References • • • • • • • Macdonald et al (1993) Pienitz (1997 a and b) Pienitz et al (1999) Pienitz, R. and Vincent , W. (2000) Effects of climate change relative to ozone depletion on UV exposure in subarctic lakes. Nature, Vol. 404, pp. 484-487. Schindler, D., Curtis, P.J., parker, B. and Stainton, M. (1996) Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature, Vol. 379, pp. 705-708. Schindler (1998) Schindler (2001) References • • • • • • Smol, J.P., Walker, I.R. and Leavitt, P.R. (1991) paleolimnology and hindcasting climatic trends. Verhandlungen der Internationalen Vereinigung von Limnologen, Vol. 24, pp. 1240-1246. Smol , J.P. and Cumming , B.F. (2000) Tracking long-term changes in climate using algal indicators in lake sediments. Journal of Phycology, Vol. 36, pp. 986-1011. Verleyen et al (2005) Vincent , W.F. and Roy, S. (1993) Solar ultraviolet-B radiation and aquatic primary production: damage, protection and recovery. Environmental Reviews, Vol. 1, pp. 1-12. Vincent , W. and Pienitz , R. (1996) Sensitivity of high-latitude freshwater ecosystems to global change: temperature and solar radiation. Geoscience Canada, Vol. 23, pp. 231-236. Yan, N.D., Keller, W., Scully, N., Lean, D. and Dillon, P. (1996) Increased UV-B penetration in a lake owing to drought-induced acidification. Nature, Vol. 381, pp. 141-143.