* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Genomic library wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Non-coding DNA wikipedia , lookup
Public health genomics wikipedia , lookup
Human genetic variation wikipedia , lookup
Human genome wikipedia , lookup
Metagenomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Pathogenomics wikipedia , lookup
Genome evolution wikipedia , lookup
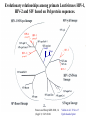
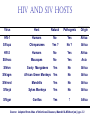
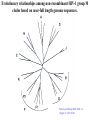
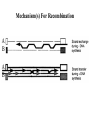
Classification of Retroviruses: • Divided into 3 subfamilies based primarily on pathogenicity rather than genome relationships. Subfamilies Oncovirinae Lentivirinae Spumavirinae Classification of Retroviruses: •Subfamilies are further divided based on: 1.Virion structure (types A-D) 2.Utilization of particular cell receptors 3.Lifestyle: whether endogenous or exogenous 4.Presence or absence of an oncogene 5.Other pathogenic properties •When nucleotide sequences and genome structure are considered, 7 groups (genus) emerge. Classification of Retroviruses: Subfamily Genus Isolates Lentivirinae Lentivirus HIV-1 HIV-2 SIV FIV Visna/maedi EIAV CAEV Lentivirus •All Retroviruses contain gag, pol, pro* and env •Lentiviruses are more complex: Two regulatory genes: tat and rev Accessory genes: HIV-1 nef, vif, vpr, vpu HIV-2 and SIV lack vpu and have vpx *pro is sometimes contained within pol (HIV) Genetic subtypes of HIV-1 Groups M O N Clades or A-H, J (9) Subtypes Limited Spread Subclades F1 &F2 Strains or isolates JR-CSF P 1 infected person Reported ‘09 2nd infected person Reported ‘10 8 full, and 5 partial, Genome sequences Genetic subtypes of HIV Groups (env sequences): M, O, N, P M: Main, O: Outlier, N: New or Non-M-Non-O, P follows O Subtypes or clades (env sequences): A-H, J Equidistant env (25-30% a.a. differences) Up to 20% a.a. sequence differences within a clade Full length gene sequences required with no evidence for recombination Strains or isolates: e.g., HIV IIIb, HIV RF Reasons For Genetic Diversity Of HIV: 1. Zoonotic transmission from at least 3 sources: Chimpanzees and Sooty Mangabees and Gorillas on at least eight different occasions. 2. Rapid rate of mutation due to: Reverse transcriptase (~1 error per genome) and fast turnover in humans. 3. Recombination events Evolutionary relationships among primate Lentiviruses HIV-1, HIV-2 and SIV based on Pol protein sequences. Group P SIVgor Peeters and Sharp AIDS 2000, 14 (Suppl 3): S129-S140. Vallari et al. J.V. Nov. 17 Epub ahead of print HIV and SIV Hosts Virus Host Natural Pathogenic Origin HIV-1 Humans No Yes Africa SIVcpz Chimpanzees Yes ? No ? Africa HIV-2 Humans No Yes Africa SIVmac Macaques No Yes Asia Yes No Africa African Green Monkeys Yes No Africa SIVsm SIVagm Sooty Mangabees SIVmnd Mandrills Yes No Africa SIVsyk Sykes Monkeys Yes No Africa SIV gor Gorillas Yes ? Africa Source: Adapted from Atlas of Infectious Diseases, Mandell & Mildvan (ed.), pp. 2.3 Cross Species Transmission of SIVcpz •SIVcpz and HIV-1 are identical in genomic organization. Only two lentiviruses to contain vpu. •Sequence of SIVcpz suggests HIV-1 transmission from P. t. troglodytes. •Observed natural infection of P. t. troglodytes with isolates more closely related to SIVcpzUS and YBF30 (group N) than any other HIV-1/SIV. Origins of HIV-1 •West Equatorial Africa is the only location where M, N, and co-circulate, and where P. t. troglodytes is infected with closely related viruses. •Group M appears to originate from SIVcpzPtt in South Central Cameroon. •Group N most likely originated in southeastern Cameroon as N sequences are highly related to SIVcpzPtt in that region. Origins of HIV-1 • Infection of Gorillas and Chimpanzees in Cameroon with viruses closely related to Group O HIV. • Although sequences suggest chimpanzee transmission to Gorillas, it’s unclear which animal is responsible for transmission to humans. • Gorilla transmission to one woman in Cameroon resulted in group P • Greatest diversity of group M is observed in Cameroon. Diversity resulting from base misincorporation (clades: resemble each other across the genome) Reverse transcriptase • Lacks proofreading function • 1000-fold higher rate of nucleotide substitutions than seen with replication of viral DNA genomes • 1 nucleotide change is introduced each time provirus is synthesized • This results in quasispecies formation Genetic subtypes of HIV Groups (env sequences): M, O, N, P M: Main, O: Outlier, N: New or Non-M-Non-O Subtypes or clades (env sequences): A-H, J Equidistant env (20-30% a.a. differences) Up to 10-15% a.a. sequence differences within a clade Full length gene sequences required with no evidence for recombination Strains or isolates: e.g., HIV IIIb, HIV RF Group M (1959 [1910 – 1950]) •Arose from one cross-species transmission event. •Most prominent group worldwide with 11 subtypes or clades: A-K •Of these only 9 are true clades, E and I appear to be recombinants. •G clade has accessory genes that resemble clade A but it is otherwise distinct. •Are some unclassified clades. Group M cont. •Has four subclades: F1, F2, B and D •F diverged into F1 and F2 and divergence between these two subclades is not much greater than between other clades. •B and D show the same extent of divergence but for historical reasons continue to be classified as clades, not subclades. •Clade B is the most common clade in North America and Europe. Evolutionary relationships among non-recombinant HIV-1 group M clades based on near-full length genome sequences. Peeters and Sharp AIDS 2000, 14 (Suppl 3): S129-S140. Spira et al. J. of Antimicrobial Chemotherapy 2003, 51: 229-240 Group O (1963) •First described in ~1990 in Camaroon, France and Gabon •Highly divergent from group M with only ~50% homology to M in env. •Represents a minority of infections in those regions, ~2-5%. •Has no subtypes which may be the result of slow and limited spread, although with more sequencing of genomes, subtypes may be found. Group N •Recently identified (1995) •~12 confirmed infections all in Camaroon •Sequences suggest this group is mosaic •5’ half distantly related to M, 3’ half more closely resembles SIVcpzus Group P • 1st known infection (seropositive 2004, reported 2009) • Woman in Paris, France recently emigrated from Cameroon • Sequences suggest SIVgor was responsible • Cannot detect by PCR using M or O primers Group P • 2nd individual identified in Camerron • HIV-seropositive male hospital patient • Screening of 1,736 HIV-seropositive suggest group is rare ~ only 0.06% of HIVinfections • Reported 2010 Vallari et al. J.V. Nov. 17th Epub ahead of print Diversity due to recombination (mosaics: genes derived from more than one parental strain) Mechanism(s) For Recombination A B A B Recombinants or Mosaics •Eg. MAL: one of the first African HIV-1 isolates characterized. •1994: first multiply infected individual identified. • ~10-20% of newly characterized strains. •Identified by discrete breakpoints in genomic regions. •Isolated in regions where both parental strains are found. Recombinants or Mosaics (cont.) •Similar breakpoints reflect common ancestry. •Designated ‘circulating recombinant forms: (CRF) •CRFs are given identifying numbers followed by letters reflecting the parental clades: CRF01-AE •If more than three clades are parental, the CRF is followed by cpx for ‘complex’. CRF01-CPX Evolutionary relationships of CRFs, CRF01-AE and CRF02-AG. Based on full length gag, 3’ end of pol, and gp120 sequences. Peeters and Sharp AIDS 2000, 14 (Suppl 3): S129-S140. Evolutionary relationships of CRFs, CRF01-AE and CRF02-AG. Based on full length gag, 3’ end of pol, and gp120 sequences. Peeters and Sharp AIDS 2000, 14 (Suppl 3): S129-S140. Geographical distribution of predominant groups, clades and CRF’s. Peeters and Sharp AIDS 2000, 14 (Suppl 3): S129-S140. Importance of CRFS •Successful transmission of SIV to Chimpanzees and humans, and subsequent human to human transmission, required viral adaptation. •Recombination increases the odds of “successful diversity”. Indeed, transmission of SIV to Chimpanzees is thought to have included a recombinant event. •CRF01_AE is Southeast Asia and CRF02_AG in West Africa are the fastest-spreading epidemic strains. •Subtypes A, C and CRF02_AG now account for 75% of the 14,000 estimated new daily infections worldwide. Heeney et al. Science 2006, 313:462 Why is understanding diversity important? •Key to understanding the path of the epidemic. •Diagnostic tests: mainly available for clade B, and diversity can affect sensitivity. •Antiretroviral drugs: mainly available for clade B only. Group O and HIV-2 are naturally resistant to NNRTs. Group M shows variation in drug susceptibilities. •Transmission and rates of disease progression differ between HIV-1 and HIV-2. Why is understanding diversity important? •Biological differences among groups and clades: Virus or host factors? •Vaccines: nAb and CTL may be clade specific – or not. Implications are that conserved regions must be included. •Vaccines and gene therapy: Recombination events are a concern. Endogenous retroviruses and infectious viruses must be considered. So, How Did Chimpanzees Get Infected? Chimpanzees are predatory animals. Prey of chimpanzees are also infected with SIV Infection was with a recombinant virus, an ancestor of viral strains currently infecting red-capped Mangabeys and the greater spot-nosed monkeys. Both of these monkeys have overlapping ranges with P.t. troglodytes in west and central Africa. Origin of infection of P. t. schweinfurthii is not currently clear. So, How Did gorillas Get Infected? Gorillas are not predatory animals. Share home range with chimpanzees who are also infected with SIV Route of infection is currently unclear More References: 1. Hybrid origin of SIV in Chimpanzees Bailes et al. Science 2003, 300:1713 1. Origins of HIV and the Evolution of Resistance to AIDS. Heeney et al. Science 2006, 313:462 3. The Black Death and AIDS: CCR5-D32 in genetics and history Q.J.Med 2006, 99:497 4. Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1 Keele et al. Science 2006, 313:523 More References • Molecular Epidemiology of Simian Immunodeficiency Virus Infection in Wildliving Gorillas Neel C, Etienne L, Li Y, Takehisa J, Rudicell RS, et. Al. J Virol. 2009 Nov 11. [Epub ahead of print]PMID: 19906908 [PubMed - as supplied by publisher] • Origin and Biology of Simian Immunodefiency Virus in Wild-Living Western Gorillas Takehisa J, Kraus MH, Ayouba A, Bailes E, Van Heuverswyn F, Decker JM, Li Y, Rudicell RS, Learn GH, Neel C, Ngole EM, Shaw GM, Peeters M, Sharp PM, Hahn BH. J Virol. 2009 Feb;83(4):1635-48. Epub 2008 Dec 10.PMID: 19073717