* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 4 Marine Sedimentation
Marine biology wikipedia , lookup
Arctic Ocean wikipedia , lookup
Oceanic trench wikipedia , lookup
Marine geology of the Cape Peninsula and False Bay wikipedia , lookup
Marine pollution wikipedia , lookup
Ocean acidification wikipedia , lookup
Anoxic event wikipedia , lookup
Physical oceanography wikipedia , lookup
Abyssal plain wikipedia , lookup
Ecosystem of the North Pacific Subtropical Gyre wikipedia , lookup
Blue carbon wikipedia , lookup
Marine Sedimentation
• Sediment Defined:
• unconsolidated
organic and
inorganic particles
that accumulate on
the ocean floor
• originate from
numerous sources
– weathering and
erosion of the
continents
– volcanic eruptions
– biological activity
– chemical processes
within the oceanic
crust and seawater
– impacts of extraterrestrial objects
• classified by size
according to the
Wentworth scale
• grain size indicates condition under which sediment is
deposited
– high energy environments characteristically yield sediments larger
in size
– small particles (silts, clays) indicate low energy environments
• considered well-sorted if most particles appear in the
same size classification
• poorly sorted sediments comprised of multiple sizes
• sediment maturity is indicated by several factors
– decreased silt and clay content
– increased sorting
– increased rounding of grains, as a result of weathering and
abrasion
• particle transport is controlled by grain size and velocity
of transporting medium
•
• Average grain
size reflects
the energy of
the
depositional
environment.
• Hjulstrom’s
Diagram
graphs the
relationship
between
particle size
and energy for
erosion,
transportation
and
deposition.
4-1
Sediment in the Sea
Classification of
marine
sediments can
be based upon
size or origin.
• Size classification
divides sediment by
grain size into gravel,
sand and clay.
– Mud is a mixture of silt
and clay.
• Origin classification
divides sediment into
five categories:
Terrigenous
sediments, Biogenic
sediments, Authigenic
sediments,
Volcanogenic
sediments and
Cosmogenic
sediments.
4-1
Sediment in the Sea
• Terrigenous (or Lithogenous
Sediments):
• derived from weathering of
rocks at or above sea level
(e.g., continents, islands)
• two distinct chemical
compositions
– ferromagnesian, or iron-magnesium
bearing minerals
– non-ferromagnesian minerals – e.g.,
quartz, feldspar, micas
• largest deposits on continental
margins (less than 40% reach
abyssal plains)
• transported by water, wind,
gravity, and ice
• transported as dissolved and
suspended loads in rivers,
waves, longshore currents
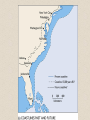
• (LANDSAT images
adapted from Geospace
Images catalog).
• sediment delivered to
the open-ocean by wind
activity as particulate
matter (dust)
• primary dust source is
deserts in Asia and
North Africa
• comprise much of the
fine-grained deposits in
remote open-ocean
areas (red clays)
• volcanic eruptions
contribute ash to the
atmosphere which
settles within the
oceans
• sediment also
transported to the
open-ocean by gravitydriven turbidity
currents
• dense 'slurries' of
suspended sediment
moved as turbulent
underflows
• typically initiated by
storm activity or
earthquakes
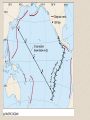
– first identified during
1929 Grand Banks
earthquake
– seismic activity triggered
turbidity current which
severed telegraph lines
• initial flow often
confined to submarine
canyons of the
continental shelf and
slope
• form deep-sea fans
where the mouth of the
canyon opens onto the
continental rise
20 m s-1
near Grand
Banks
• boulder to clay size
particles also eroded
and transported to
oceans via glacial ice
• glacier termination in
circum-polar oceans
results in calving and
iceberg formation
• as ice (or icebergs)
melt, entrained
material is deposited
on the ocean floor
• termed 'ice-rafted'
debris
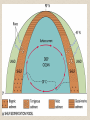
• Biogenous Sediments:
• composed primarily of
marine microfossil
remains
• shells of one-celled
plants and animals,
skeletal fragments
• median grain size
typically less than 0.005
mm (i.e., silt or clay size
particles)
• characterized as CaCO3
(calcium carbonate) or
SiO2 (silica) dominated
systems
• sediment with biogenic
component less than
30% termed calcareous,
siliceous clay
• calcareous or siliceous
'oozes' if biogenic
component greater than
30%
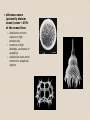
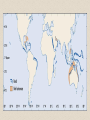
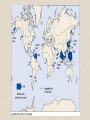
• siliceous oozes
(primarily diatom
oozes) cover ~15%
of the ocean floor
– distribution mirrors
regions of high
productivity
– common at high
latitudes, and zones of
upwelling
– radiolarian oozes more
common in equatorial
regions
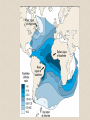
• calcareous oozes
(foraminifera,
coccolithophores) cover
~50% of the ocean floor
– distribution controlled
largely by dissolution
processes
– cold, deep waters are
undersaturated with respect
to CaCO3
– deep water is slightly acidic
as a result of elevated CO2
concentrations
– solubility of CaCO3 also
increases in colder water
and at greater pressures
– CaCO3 therefore readily
dissolved at depth
• level below which no CaCO3
is preserved is the
'carbonate compensation
depth'
• typically occurs at a depth
of 3000 to 4000 m
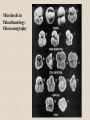
•
Microfossils in
Paleoclimatology/
Paleoceanography
• Dissolution
Calcium carbonate
dissolves better in
colder water, in acidic
water, and at higher
pressures. In the deep
ocean, all three of these
conditions exist.
Therefore, the
dissolution rate of
calcium carbonate
increases greatly below
the thermocline. This
change in dissolution
rate is called the
lysocline.
Below the lysocline,
more and more calcium
carbonate dissolves,
until eventually, there
is none left. The depth
below which all calcium
carbonate is dissolved
is called the carbonate
compensation depth or
CCD.
• Hydrogenous (or Authigenic) Sediments:
• produced by chemical processes in seawater
• essentially solid chemical precipitates of several common
forms
• non-biogenous carbonates
– form in surface waters supersaturated with calcium carbonate
– common forms include short aragonite crystals and oolites
• phosphorites
– phosphate crusts (containing greater than 30% P2O5) occurring
as nodules
– formed as large quantities of organic phosphorous settle to the
ocean floor
– unoxidized material is transformed to phosphorite deposits
– found on continental shelf and upper slope in regions of high
productivity
• manganese
nodules
– surficial
deposits of
manganese,
iron, copper,
cobalt, and
nickel
– accumulate
only in areas
of low
sedimentation
rate (e.g., the
Pacific)
– develop
extremely
slowly (1 to 10
mm/million
years)
•
• The term evaporites is
used for all deposits, such
as salt deposits, mainly
chemical sediments that
are composed of minerals
that precipitated from
saline solutions
concentrated by
evaporation. Evaporite
deposits are composed
dominantly of varying
proportions of halite (rock
salt) (NaCl), anhydrite
(CaSo4) and gypsum
(CaSo4.2H2O). Evaporites
may be classified as
chlorides, sulfates or
carbonates on the basis of
their chemical composition
(Tucker, 1991).
evaporites ('salt'
deposits')
occur in regions
of enhanced
evaporation
(e.g., marginal
seas)
evaporative
process removes
water and leaves
a salty brine
e.g.,
Mediterranean
'Salinity Crisis'
between 5 and 6
million years
• Cosmogenous
Sediments:
• sediments derived
from
extraterrestrial
materials
• includes
micrometeorites
and tektites
• tektites result from
collisions with
extraterrestrial
materials
– fragments of earth's
crust melt and spray
outward from impact
crater
– crustal material remelts as it falls back
through the
atmosphere
– forms 'glassy' tektites
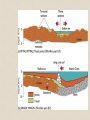
• Distribution of Marine
Sediments:
• sediments thickest along
continental margins, thin
at mid-ocean ridges
• coastlines
– dominated by river-borne and
wave reworked terrigenous
sediments
– shelf and slope characterized
by turbidites and authigenic
carbonate deposits
– glacial deposits and ice-rafted
debris common at high
latitudes
– high input of terrigenous
sediments 'dilutes' biogenous
components
• deep-sea (pelagic) basins
– abyssal clays (wind blown
deposits) common
– lower quantities of biogenic
material
• distribution of biogenous
sediments dependent
upon three primary factors
– production in surface waters
– dissolution in deep waters
– dilution by other sediments
types
• high productivity in
zones of upwelling and
nutrient-rich high
latitude waters
• calcareous oozes more
common in warmer or
shallower water
• siliceous oozes more
common in colder or
deeper water
• terrigenous
sedimentation rates
range from ~1 mm to
10's cm/1000 years
• biogenous
sedimentation rates
typically ~1 cm/1000
years
Nearshore sediments, turbidites:Up to
km/my (kilometers/million years)
Hemipelagic deposits: Tens to hundreds
of m/myDrift deposits40-400 m/my
Mid-latitude eolian deposits: 3 to 10
m/my
Ice rafted material: 10+ m/my
Carbonate oozes: Up to 50 m/my
Siliceous oozes: Up to 10 m/my
Hydrothermal deposits: (off ridge
axes)About 0.5 m/my
Hydrogenous sediments: Rarely exceed
0.2 m/my
Ferromanganese nodules: 0.0002 to
0.005 m/my (0.2 to 5 mm/my)
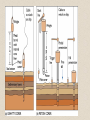
Shelf sedimentation4-2
is
strongly controlled by
tides, waves and
currents, but their
influence decreases
with depth.
• Shoreline turbulence
prevents small particles
from settling and transports
them seaward where they
are deposited in deeper
water.
• Particle size decreases
seaward for recent
sediments.
• Past fluctuations of sea
level has stranded coarse
sediment (relict sediment)
across the shelf including
most areas where only fine
sediments are deposited
today.
Sedimentation in the Ocean
4-2
Sedimentation in the Ocean
Geologic controls of continental shelf
sedimentation must be considered in terms of a
time frame.
• For a time frame up to 1000 years, waves, currents and
tides control sedimentation.
• For a time frame up to 1,000,000 years, sea level lowered
by glaciation controlled sedimentation and caused rivers to
deposit their sediments at the shelf edge and onto the
upper continental slope.
• For a time frame up to 100,000,000 years, plate tectonics
has determined the type of margin that developed and
controlled sedimentation.
60% of the
world’s shelves
are covered
with relict
sediments that
were formed
about 15,000 y
BP under a
different energy
regime.
• Gas Methane Hydrates
(Clathrates)
• Hydrates store immense
amounts of methane, with
major implications for
energy resources and
climate, but the natural
controls on hydrates and
their impacts on the
environment are very
poorly understood
• The worldwide amounts of
carbon bound in gas
hydrates is conservatively
estimated to total twice the
amount of carbon to be
found in all known fossil
fuels on Earth (USGS).
• Methane bound in hydrates
amounts to approximately
3,000 times the volume of
methane in the
atmosphere.