* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Periodic Table - Harlan Independent Schools
Electrical resistivity and conductivity wikipedia , lookup
Drug discovery wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Coordination complex wikipedia , lookup
Geochemistry wikipedia , lookup
Condensed matter physics wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Atomic orbital wikipedia , lookup
Organic chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Livermorium wikipedia , lookup
Chemical bond wikipedia , lookup
Bond valence method wikipedia , lookup
Electronegativity wikipedia , lookup
History of molecular theory wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Boron group wikipedia , lookup
Chemical element wikipedia , lookup
Electron configuration wikipedia , lookup
Metallic bonding wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
History of chemistry wikipedia , lookup
Alkaline earth metal wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Atomic theory wikipedia , lookup
Periodic table wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
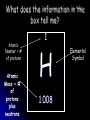
Bell Ringer Why is Mendeleev important? Bell Ringer NOTES--- Periodic Table arrangement Families of the periodic table The Periodic Table Unit II—Part 1 The Father of the Periodic Table— Dimitri Mendeleev Mendeleev was the first scientist to notice the relationship between the elements Arranged his periodic table by atomic mass Said properties of unknown elements could be predicted by the properties of elements around the missing element Moseley later discovered that the periodic nature of the elements was associated with atomic number, not atomic mass EXAMPLES OF PHYSICAL PROPERTIES - Density - Boiling Point - Melting Point - Conductivity - Heat Capacity Trends EXAMPLES OF CHEMICAL PROPERTIES - Valence - Reactivity - Radioactivity The Periodic Table Column = Group or Family 18 columns on the Periodic Table Row = Period 7 rows on the Periodic Table What does the information in the box tell me? Atomic Number = # of protons Atomic Mass = # of protons plus neutrons 1 H 1.008 Elemental Symbol Elemental Symbol All elements have their own unique symbol. It can consist of a single capital letter, or a capital letter and one or two lower case letters. Columns of elements are called groups or families. Elements in each family have similar but not identical properties. For example, lithium (Li), sodium (Na), potassium (K), and other members of family IA are all soft, white, shiny metals. All elements in a family have the same number of valence electrons. Each horizontal row of elements is called a period. The elements in a period are not alike in properties. In fact, the properties change greatly across even given row. The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas. Metals, Nonmetals, and Only Semi-metals Nonmetals are on the nonmetal on the metal side Metals are to the left of the stair- step Semi-metals, “metalloids,” touch the stair-step right of the stair-step Hydrogen The hydrogen square sits atop Family AI, alkali metals ; but it is not a member of that family. Hydrogen is in a class of its own. It’s a gas at room temperature. It has one proton and one electron in its one and only energy level. Hydrogen only needs 2 electrons to fill up its valence shell. Group 1: Alkali Metals Most reactive metals on the PT Rarely found free in nature usually found in compounds Charge of 1—1 valence electron Except Hydrogen Members(Na, Li,K, Rb, Cs,Fr) They are shiny, have the consistency of clay, and are easily cut with a knife Group 2: Alkaline Earth Metals Charge of 2—2 valence electrons Members(Be, Mg, Ca,Sr,Ba, and Ra) . This is the second most reactive family of elements in the periodic table. Did you know why they are called alkaline? When these compounds are mixed in solutions, they are likely to form solutions with a pH greater than 7. Those pH levels are defined as 'basic' or 'alkaline' solutions. As you get to the bottom of the list, you will find the radioactive radium (Ra). While radium is not found around your house anymore, it used to be used in glow-in-thedark paints. The other elements are found in many items including fireworks, batteries, flashbulbs, and special alloys. The lighter alkaline earth metals such as magnesium and calcium are very important in animal and plant physiology. You all know that calcium helps build your bones. Groups 3-12: Transition Metals Found freely and in compounds in nature Charge is usually 2 but can vary— usually 2 valence electrons These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. Transistion (continued) Transition elements have properties similar to one another and to other metals, but their properties do not fit in with those of any other family. Many transition metals combine chemically with oxygen to form compounds called oxides. Group 13: Boron Family The Boron Family is named after the first element in the family. Atoms in this family have 3 valence electrons. This family includes a metalloid (boron), and the rest are metals. This family includes the most abundant metal in the earth’s crust (aluminum). Group 14: The Carbon Family Contains elements that can form unusual bonds (carbon and silicon) Charge is +4 or -4—contains 4 valence electrons This family includes a non-metal (carbon), metalloids, and metals. The element carbon is called the “basis of life.” There is an entire branch of chemistry devoted to carbon compounds called organic chemistry. Group 15: The Nitrogen Family Charge is -3—contains 5 valence electrons The nitrogen family is named after the element that makes up 78% of our atmosphere. This family includes non-metals, metalloids, and metals. They tend to share electrons when they bond. Other elements in this family are phosphorus, arsenic, antimony, and bismuth. Group 16: The Oxygen Family Also known as the chalcogens Charge is -2 Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. Group 17: The Halogens Most reactive nonmetals charge is -1—7 valence electrons Halogen atoms only need to gain 1 electron to fill their outermost energy level. They react with alkali metals to form salts. The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. Halogens have 7 valence electrons, which explains why they are the most active non-metals. They are never found free in nature Group 18: The Noble Gases (The Inert Gases) Nonreactive Charge is 0—2 or 8 valence electrons Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's atmosphere. Special Rows on the PT Lanthanides Actinides Lanthanides and Actinides The thirty rare earth elements are composed of the lanthanide and actinide series. One element of the lanthanide series and most of the elements in the actinide series are called trans-uranium, which means synthetic or man-made. All matter is composed of atoms and groups of atoms bonded together, called molecules. Substances that are made from one type of atom only are called pure substances. Substances that are made from more than one type of atom bonded together are called compounds. Compounds that are combined physically, but not chemically, are called mixtures. Elements, Compounds, Mixtures Sodium is an element. Chlorine is an element. When sodium and chlorine bond they make the compound sodium chloride, commonly known as table salt. Elements, Compounds, Mixtures Mixtures can be separated by physical means. Compounds can only be separated by chemical means. Elements are pure substances. When the subatomic particles of an element are separated from its atom, it no longer retains the properties of that element. Elements, Compounds, Mixtures Hydrogen is an element. Oxygen is an element. When hydrogen and oxygen bond they make the compound water. When salt and water are combined, a mixture is created. Compounds in mixtures retain their individual properties. The ocean is a mixture.