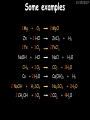* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Structures and Bonding
Stoichiometry wikipedia , lookup
History of electrochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Biological aspects of fluorine wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Atomic theory wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Sodium bicarbonate wikipedia , lookup
Acid strength wikipedia , lookup
Geochemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Biochemistry wikipedia , lookup
Sodium hypochlorite wikipedia , lookup
Sodium hydroxide wikipedia , lookup
Water splitting wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Electrochemistry wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Electrolysis of water wikipedia , lookup
Acid–base reaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
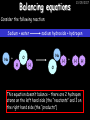
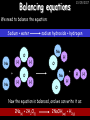
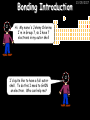
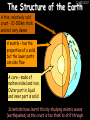
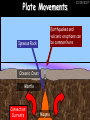
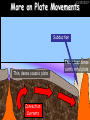
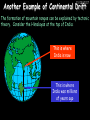
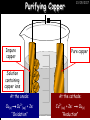
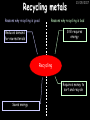
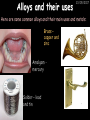
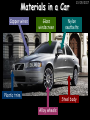
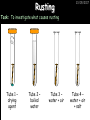
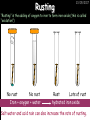
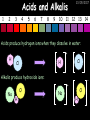
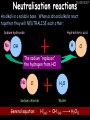
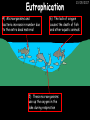
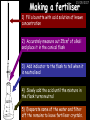
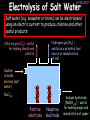
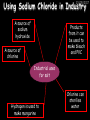
23/05/2017 C2 Chemical Resources OCR Gateway Additional Science W Richards The Weald School Fundamental Concepts 23/05/2017 Chemical formulae 23/05/2017 The chemical formulae of a molecule or compound is simply a way of showing the ratio of atoms in it. For example… Na Cl = sodium chloride (NaCl) K I = potassium iodide (KI) O K N O O = potassium nitrate (KNO3) Chemical formulae Try drawing these: 1) Water H2O 2) Carbon dioxide CO2 3) Calcium sulphate CaSO4 4) Magnesium hydroxide Mg(OH)2 23/05/2017 Naming compounds 23/05/2017 Rule 1– If two identical elements combine then the name doesn’t change This happens with the following elements: 1) H2 4) F2 2) N2 5) Cl2 3) O2 6) Br2 Naming compounds 23/05/2017 Rule 2 – When two elements join and one is a halogen, oxygen or sulphur the name ends with ____ide e.g. Magnesium + oxygen magnesium oxide 1) Sodium + chlorine 6) KBr 2) Magnesium + fluorine 7) LiCl 3) Lithium + iodine 8) CaO 4) Chlorine + copper 9) MgS 5) Oxygen + iron 10)KF Naming compounds 23/05/2017 Rule 3 – When three or more elements combine and two of them are hydrogen and oxygen the name ends with hydroxide e.g. Sodium + hydrogen + oxygen Sodium hydroxide 1) Potassium + hydrogen + oxygen 2) Lithium + hydrogen + oxygen 3) Calcium + hydrogen + oxygen 4) Mg(OH)2 Naming compounds 23/05/2017 Rule 4 – When three or more elements combine and one of them is oxygen the ending is _____ate e.g. Copper + sulphur + oxygen Copper sulphate 1) Calcium + carbon + oxygen 6) AgNO3 2) Potassium + carbon + oxygen 7) H2SO4 3) Calcium + sulphur + oxygen 8) K2CO3 4) Magnesium + chlorine + oxygen 5) Calcium + oxygen + nitrogen Simple formulae to learn 23/05/2017 H2O Water NaCl Sodium chloride CO2 Carbon dioxide CaCl2 Calcium chloride NH3 Ammonia MgO Magnesium oxide H2 Hydrogen HCl Hydrochloric acid O2 Oxygen NaOH Sodium hydroxide N2 Nitrogen Ca(OH)2 Calcium hydroxide K2SO4 Potassium sulfate CaCO3 Calcium carbonate Cl2 Chlorine HNO3 Nitric acid KCl Potassium chloride H2SO4 Sulphuric acid CuO Copper oxide Mg(OH)2 Magnesium hydroxide Na2CO3 Sodium carbonate MgCl2 Magnesium chloride (NH4)2SO4 Ammonium sulfate Simple chemical reactions 23/05/2017 H Mg O Cl H Cl Cl Magnesium + Hydrochloric oxide acid MgO + 2HCl Mg H Cl Magnesium chloride + Water MgCl2 + H2O O O Mg Cu O S O Cu Mg Mg + Copper sulphate CuSO4 O S O O O Magnesium + O H Copper + Magnesium sulphate Cu + MgSO4 Notice that the number of atoms on each side of the equation is the same! Balancing equations 23/05/2017 Consider the following reaction: Sodium + water Na + sodium hydroxide + hydrogen Na O H H O H + H H This equation doesn’t balance – there are 2 hydrogen atoms on the left hand side (the “reactants” and 3 on the right hand side (the “products”) Balancing equations 23/05/2017 We need to balance the equation: Sodium + water sodium hydroxide + hydrogen Na O H Na + Na H O O H Na H O H + H H Now the equation is balanced, and we can write it as: 2Na(s) + 2H2O(l) 2NaOH(aq) + H2(g) H Some examples 2Mg O2 2 MgO Zn + 2 HCl ZnCl2 2 Fe + 3Cl2 2 FeCl3 NaOH CH4 Ca + + HCl + 2 O2 NaCl CO2 + + 23/05/2017 H2 H 2O + 2H2O + 2 H2O Ca(OH)2 + + H2SO4 Na2SO4 + 2H2O 2 CH3OH + 3 O2 2 NaOH 2 CO2 + 4H2O H2 Bonding Introduction Cl Hi. My name’s Johnny Chlorine. I’m in Group 7, so I have 7 electrons in my outer shell I’d quite like to have a full outer shell. To do this I need to GAIN an electron. Who can help me? Cl 23/05/2017 Ionic Bonding Here comes my friend again, Sophie Sodium Cl Na Hey Johnny. I’m in Group 1 so I have one electron in my outer shell. Unlike Harry, this electron is far away from the nucleus so I’m quite happy to get rid of it. Do you want it? Okay + Cl 23/05/2017 Na Now we’ve both got full outer shells and we’ve both gained a charge. We’ve formed an IONIC bond, which is basically caused by the attraction between our charges. Covalent Bonding Cl 23/05/2017 Here comes another one of my friends, Harry Hydrogen Hey Johnny. I’ve only got one electron but it’s really close to my nucleus so I don’t want to lose it. Fancy sharing? Cl H Now we’re both really stable. We’ve formed a covalent bond. H Balancing ions Some common ions: Sodium – Na+ Chloride – Cl- Potassium – K+ Bromide – Br- Magnesium – Mg2+ Oxide – O2- Ammonium – NH4+ Sulphate – SO42- Determine the formula of the following compounds: 1) Sodium chloride 2) Magnesium oxide 3) Magnesium chloride 4) Ammonium chloride 5) Sodium sulphate 6) Sodium oxide 23/05/2017 Atom, molecule or ion? Are the following things atoms, molecules or ions? 1) H2 1) Molecule 2) NH3 2) Molecule 3) Cl- 3) Ion 4) K2SO4 4) Molecule 5) Au 5) Atom 6) CO32- 6) Ion 7) Na 7) Atom 8) CO2 8) Molecule 9) H+ 9) Ion 10)H2O 10)Molecule 23/05/2017 C2a The Structure of the Earth 23/05/2017 The Structure of the Earth 23/05/2017 A thin, relatively cold crust - 10-100km thick and not very dense A mantle – has the properties of a solid but the lower parts can also flow A core – made of molten nickel and iron. Outer part is liquid and inner part is solid Scientists have learnt this by studying seismic waves (earthquakes) as the crust is too think to drill through. Movement of the Lithosphere 23/05/2017 The Earth’s LITHOSPHERE (i.e. the _______) is split up into different sections called ________ plates: These plates are moving apart from each other a few centimetres every _______ due to the ________ currents in the mantle caused by the ________ decay of rocks inside the core. Words – radioactive, crust, convection, tectonic, year Plate Movements Earthquakes and volcanic eruptions can be common here Igneous Rock Oceanic Crust Mantle Convection Currents 23/05/2017 Magma More on Plate Movements 23/05/2017 Subduction Thin, dense oceanic plate Convection Currents Thick, less dense continental plate Tectonic Plate movements 23/05/2017 Look at the coastlines of South America and Africa. I wonder of they used to fit together… Alfred Wegener I’m going to call this my Theory of Continental Drift Tectonic theory What’s my evidence for this? Three things: 1) The “jigsaw fit” 2) Each continent has similar rocks and fossils 3) Each continent has similar animal species 23/05/2017 The Evidence: Tectonic theory 23/05/2017 1) Some continents look like they used to “fit” together 2) Similar rock patterns and fossil records The Problems: Wegener couldn't explain how continental drift happened so nobody believed him The Answer: 1) Scientists discovered 50 years later that the Earth generates massive amounts of heat through radioactive decay in the core. This heat generated convection currents in the mantle causing the crust to move 2) We also now know that the sea floor is spreading outwards from plate boundaries Conclusion – scientists now believe Wegener’s Tectonic 23/05/2017 Another Example of Continental Drift The formation of mountain ranges can be explained by tectonic theory. Consider the Himalayas at the top of India: This is where India is now This is where India was millions of years ago 23/05/2017 Magnetic Patterns in Sea Floor Spreading The Earth’s magnetic field swaps poles every million years. The above picture shows those changes recorded over time in rocks on the sea floor and provides evidence for long-term sea floor spreading. Igneous Rock 23/05/2017 Granite – a slow cooling rock with big crystals and rich in silica Rhyolite – a fast cooling rock with small crystals and rich in silica Basalt – a fast cooling rock with small crystals and rich in iron Gabbro – a slow cooling rock with big crystals and rich in iron Volcanoes 23/05/2017 What do you think of the following photo? Picture: EPA Geologists study volcanoes to get better at forecasting future ones and to reveal more information about the structure of the Earth. C2b Construction Materials 23/05/2017 Building Materials 23/05/2017 Many of our common building materials are found in the Earth: Iron and aluminium come from ores. Glass is made from sand Bricks are made from clay Building Materials 23/05/2017 Here are some common rocks used in buildings: Limestone – a sedimentary rock so fairly soft Marble (made from chalk or limestone under high pressure and heat) and fairly hard Granite – an igneous rock and very hard Limestone 23/05/2017 Limestone is a __________ rock made up of mainly calcium _______. It’s cheap and easy to obtain. Some facts: 1) Building materials – limestone can be quarried and cut into blocks to be used in buildings. However, it is badly affected by ____ ____. 2) Limestone ________ when heated to form calcium oxide and carbon dioxide: Calcium carbonate CaCO3 HEAT HEAT calcium oxide + carbon dioxide CaO + CO2 3) Cement making – limestone can be “roasted” in a rotary kiln to produce dry cement. It’s then mixed with sand and aggregate to make _______. Words – concrete, acid rain, carbonate, sedimentary, decomposes 23/05/2017 Pros and Cons of quarrying limestone Reasons why quarrying limestone is a good idea Reasons why quarrying limestone is a bad idea Concrete 23/05/2017 Concrete is a strong building material and can be made even stronger with reinforcements: Reinforcing concrete is better than plain concrete because it is both stronger and the steel is more flexible. C2c Metals and Alloys 23/05/2017 Extracting Metals 23/05/2017 Some definitions: A METAL ORE is a mineral or mixture of minerals from which it is “economically viable” to extract some metal. Most ores contain METAL OXIDES (e.g. rust = iron oxide). To “extract” a metal from a metal oxide we need to REDUCE the oxygen. This is called a REDUCTION reaction. To put it simply: Iron ore Iron Oxide “Reduce” the oxygen to make iron How do we do it? Potassium Sodium Calcium Magnesium Aluminium Carbon Zinc 23/05/2017 Metals ABOVE CARBON, because of their high reactivity, are extracted by ELECTROLYSIS Metals BELOW CARBON are extracted by heating them with carbon in a BLAST FURNACE. This is a “displacement reaction” Iron Tin Carbon Copper Oxide Lead Copper Silver Gold Platinum These LOW REACTIVITY metals won’t need to be extracted because they are SO unreactive you’ll find them on their own, not in a metal oxide Reducation and Oxidation 23/05/2017 OIL RIG Oxidation is Loss of Electrons Reduction is Gain of Electrons An example of reduction: Aluminium + iron oxide 2Al(s) + Fe2O3(s) heat aluminium oxide + iron heat Al2O3(s) + 2Fe(s) An example of oxidation: Magnesium + oxygen Mg(s) + O2(s) heat heat magnesium oxide 2MgO(s) Purifying Copper Impure copper Solution containing copper ions + + Cu + Cu + Cu At the anode: Cu(s) Cu2+(aq) + 2e- “Oxidation” 2+ 2+ 2+ - 23/05/2017 Pure copper At the cathode: Cu2+(aq) + 2e- “Reduction” Cu(s) Recycling metals Reasons why recycling is good 23/05/2017 Reasons why recycling is bad Still requires energy Reduces demand for raw materials Recycling Requires money to sort and recycle Saves energy Alloys 23/05/2017 Steel is an “alloy” – i.e. a mixture of metals. Here are other alloys: Gold mixed with copper Aluminium mixed with magnesium and copper Aluminiun mixed with chromium Alloys and their uses 23/05/2017 Here are some common alloys and their main uses and metals: Brass – copper and zinc Amalgam mercury Solder – lead and tin Smart Alloys 23/05/2017 A “smart alloy” is one that can “remember” its original state after being bent or stretched. These glasses are made from a “smart” material – if they are bent they will return to their original shape Gold alloys 23/05/2017 Gold can be mixed with other metals to make alloys with different properties. For example: 24-Carat gold 9-Carat gold “Pure gold” – 99.99% of the atoms in this bar are gold atoms (fineness off 999.9). Pure and malleable but soft. “9 carat gold” – around 9/24ths of the atoms in these earrings are gold atoms. Harder than pure gold but less malleable. C2d Making Cars 23/05/2017 Materials in a Car Copper wires Glass windscreen Plastic trim 23/05/2017 Nylon seatbelts Steel body Alloy wheels Rusting 23/05/2017 Task: To investigate what causes rusting Tube 1 – drying agent Tube 2 – boiled water Tube 3 – water + air Tube 4 – water + air + salt Rusting 23/05/2017 “Rusting” is the adding of oxygen to iron to form iron oxide (this is called “oxidation”) No rust No rust Iron + oxygen + water Rust Lots of rust hydrated iron oxide Salt water and acid rain can also increase the rate of rusting. Aluminium: Iron or aluminium? 23/05/2017 Does not corrode as it does not oxidise Less dense and lighter and cars are therefore more efficient More expensive but the car is more efficient and lasts longer Iron: Cheaper than aluminium Magnetic so easily recycled Corrodes easily but also malleable Most cars are made from steel (an alloy of carbon) which is harder and stronger than iron and less likely to corrode. Recycling 23/05/2017 From 2015 law states that 95% of a car must be made from recycled material. Why recycle? 1) Less space will be needed for landfill sites 2) Recycled metals only need about 1/10th of the energy to produce compared to producing new metals 3) Recycling paper reduces the amount of water and energy needed to produce it 4) Recycled glass only needs 80% of the energy to produce compared to producing new glass 5) Recycling saves on raw materials 6) Less excavation and mining costs C2e Manufacturing Chemicals 23/05/2017 Reversible Reactions 23/05/2017 Some chemical reactions are reversible. In other words, they can go in either direction: A + B e.g. Ammonium chloride NH4Cl C + D Ammonia + hydrogen chloride NH3 + HCl If a reaction is EXOTHERMIC in one direction what must it be in the opposite direction? For example, consider copper sulphate: Hydrated copper sulphate (blue) + Heat CuSO4.5H2O Anhydrous copper sulphate (white) CuSO4 + H2O + Water Reversible Reactions 23/05/2017 When a reversible reaction occurs in a CLOSED SYSTEM (i.e. no reactants are added or taken away) an EQUILIBRIUM is achieved – in other words, the reaction goes at the same rate in both directions: A + B Endothermic reactions Increased temperature: A + B C + D C + D Exothermic reactions Increased temperature: A + B C + D More products Less products Decreased temperature: Decreased temperature: A + B C + D Less products A + B C + D More products Making Ammonia 23/05/2017 Guten Tag. My name is Fritz Haber and I won the Nobel Prize for chemistry. I came up with the Haber Process that uses nitrogen (from the air) and hydrogen (from natural gas or cracking hydrocarbons) to make ammonia: Nitrogen + hydrogen Ammonia N2 + 3H2 2NH3 Fritz Haber, 1868-1934 To produce ammonia from nitrogen and hydrogen you have to use three conditions: Nitrogen Hydrogen •High pressure •450O C •Iron catalyst Mixture of NH3, H2 and N2. This is cooled causing NH3 to liquefy. Recycled H2 and N2 Haber Process: The economics 23/05/2017 A while ago we looked at reversible reactions: Endothermic, increased temperature A + B Endothermic C + D Exothermic, increase temperature A + B Nitrogen + hydrogen Ammonia N2 + 3H2 2NH3 C + D Exothermic 1) If temperature was DECREASED the amount of ammonia formed would __________... 2) However, if temperature was INCREASED the rate of reaction in both directions would ________ causing the ammonia to form faster 3) If pressure was INCREASED the amount of ammonia formed would INCREASE because there are less molecules on the right hand side of the equation Haber Process Summary 23/05/2017 A low temperature increases the yield of ammonia but is too slow A high temperature improves the rate of reaction but decreases the yield too much A high pressure increases the yield of ammonia but costs a lot of money To compromise all of these factors, these conditions are used: Nitrogen Hydrogen •200 atm pressure •450O C •Iron catalyst Mixture of NH3, H2 and N2. This is cooled causing NH3 to liquefy. Recycled H2 and N2 Industrial Processes summary 23/05/2017 Ammonia is an important chemical as its used to make fertilisers and nitric acid. What does the cost of making any chemical depend on? What pressures and temperatures are needed Equipment needed Cost of energy Whether or not a catalyst can be used Factors affecting How much a chemical costs to make How quickly the chemical can be made Cost of materials Cost of wages How much chemical is made per day Chemical Economics 23/05/2017 Hi. We’re industrial scientists and we want to make lots of chemicals and sell them to make money. What problems would we face? Therefore we need reactions and processes that give us a pure, high percentage yield where all of the products are useful and the reactions happen quickly. Possible problems with making chemicals: 1) Reactions often produce chemicals that aren’t commercially useful or that can’t be sold 2) Reactions also need to be fast to be economical but not so fast that they’re dangerous! C2f Acids and Alkalis 23/05/2017 23/05/2017 Universal Indicator and the pH scale Universal Indicator is a mixture of liquids that will produce a range of colours to show how strong the acid or alkali is: 1 2 3 Stomach acid 4 5 Lemon juice 6 7 8 9 10 11 12 13 14 Water Soap Baking powder Oven cleaner Strong alkali Strong acid Neutral We can also use litmus paper which is red in acid or blue in alkali. Acids and Alkalis 1 2 3 4 5 6 7 8 9 10 11 23/05/2017 12 13 14 Acids produce hydrogen ions when they dissolve in water: H + Cl H Cl - Alkalis produce hydroxide ions: Na O H - + Na O H Neutralisation reactions 23/05/2017 An alkali is a soluble base. When acids and alkalis react together they will NEUTRALISE each other: Sodium hydroxide Na Hydrochloric acid H OH The sodium “replaces” the hydrogen from HCl Na Cl Sodium chloride General equation: H2O Water H+(aq) + OH-(aq) H2O(l) Cl Common acids and alkalis 23/05/2017 Acids Alkalis Hydrochloric acid, HCl Sodium hydroxide, NaOH Nitric acid, HNO3 Potassium hydroxide, KOH Sulphuric acid, H2SO4 Magnesium hydroxide, Mg(OH)2 Calcium hydroxide, Ca(OH)2 Making salts 23/05/2017 Whenever an acid and alkali neutralise each other we are left with a salt, like a chloride or a sulphate. Complete the following table: Hydrochloric acid Sodium hydroxide Potassium hydroxide Calcium hydroxide Sulphuric acid Nitric acid Sodium chloride + water Potassium sulphate + water Calcium nitrate + water Phosphoric acid Adding acid to carbonates 23/05/2017 Carbonates are compounds containing carbon and oxygen. When an acid is added to a carbonate the carbonate starts to _______. A gas called ______ _______ is produced and the acid is neutralised. Carbonates used to be used as building materials but aren’t any more because acid rain would eventually ________ the building. Words – dissolve, fizz, carbon dioxide, oxygen 23/05/2017 Reactions of metals carbonates with acid A metal carbonate is a compound containing a metal, carbon and oxygen. METAL CARBONATE + ACID Mg O O H C O H SALT + CARBON DIOXIDE + WATER Cl O Cl Cl Mg Cl C O Copy and complete the following reactions: 1) Magnesium carbonate + hydrochloric acid 2) Calcium carbonate + hydrochloric acid 3) Sodium carbonate + sulphuric acid H O H Reactions of metal oxides with acid 23/05/2017 A metal oxide is a compound containing a metal and oxide. They are sometimes called BASES. For example: Mg O Na Magnesium oxide O Al Na Al Sodium oxide Mg O H O O Aluminium oxide METAL OXIDE + ACID H O SALT + WATER Cl Cl Cl Mg Cl Copy and complete the following reactions: 1) Magnesium oxide + hydrochloric acid 2) Calcium oxide + hydrochloric acid 3) Sodium oxide + sulphuric acid H O H Acids and metal hydroxides 23/05/2017 A neutralisation reaction occurs when an acid reacts with an alkali. An alkali is a metal oxide or metal hydroxide dissolved in water. ACID + ALKALI Na O H H Cl SALT + WATER Cl Na Copy and complete the following reactions: 1) Sodium hydroxide + hydrochloric acid 2) Calcium hydroxide + hydrochloric acid 3) Sodium hydroxide + sulphuric acid 4) Magnesium hydroxide + sulphuric acid H O H Neutralisation reactions 23/05/2017 The basic equation for any neutralisation reaction is: H+(aq) + OH-(aq) H2O(l) Write word and chemical equations for the following reactions: 1) Hydrochloric acid + sodium hydroxide 2) Hydrochloric acid + potassium hydroxide 3) Nitric acid + potassium hydroxide 4) Sulphuric acid + calcium hydroxide 5) Nitric acid + copper oxide, CuO 6) Sulphuric acid + calcium carbonate, Ca(CO)3 C2g Fertilisers and Crop yields 23/05/2017 Fertilisers Population The human population is growing exponentially: 23/05/2017 Time Because of this trend it is important to increase food yields and fertilisers help us to do this. Most fertilisers are “NPK” which means they contain _________, phosphates and potassium to enhance plant ______. These minerals have to be dissolved in _____ so that they can be absorbed through _____ and carried through the ______ stream. These minerals replace essential elements used by previous crops and provide more nitrogen for plant protein. Words – transpiration, nitrogen, water, growth, roots Eutrophication 23/05/2017 One possible problem with fertilisers is eutrophication. Eutrophication is when lakes become stagnant due to careless use of fertiliser… 1) Inorganic fertilisers used on fields are washed into the lake 3) This growth causes overcrowding and many plants die due to lack of enough light or food 2) The fertiliser causes increased growth in water plants Eutrophication 4) Microorganisms and bacteria increase in number due to the extra dead material 23/05/2017 6) The lack of oxygen causes the death of fish and other aquatic animals Can’t…breathe… 5) These microorganisms use up the oxygen in the lake during respiration Eutrophication 4) Microorganisms and bacteria increase in number due to the extra dead material 23/05/2017 6) The lack of oxygen causes the death of fish and other aquatic animals 5) These microorganisms use up the oxygen in the lake during respiration Ammonium-based Fertilisers 23/05/2017 Guten tag. When ammonia dissolves in water it produces an alkaline solution: NH3 + H20 Fritz Haber, 1868-1934 NH4OH This solution can be used to make fertilisers like ammonium nitrate, ammonium phosphate and ammonium sulfate. Very useful! I won the Nobel Prize for Chemistry for my work in making ammonia as my discovery has led to increased crop yields. Making a fertiliser 23/05/2017 1) Fill a burette with acid solution of known concentration 2) Accurately measure out 25cm3 of alkali and place it in the conical flask 3) Add indicator to the flask to tell when it is neutralised 4) Slowly add the acid until the mixture in the flask turns neutral 5) Evaporate some of the water and filter off the remains to leave fertiliser crystals. C2h Chemicals from the sea 23/05/2017 Salt Salt (sodium chloride, NaCl) is an important chemical and is used as a preservative and as a flavouring. Salt can be obtained from seawater, salt deposits or from mining: What problems could salt mining bring? 23/05/2017 Testing for Chlorine Chlorine “bleaches” damp indicator paper 23/05/2017 Electrolysis of Salt Water 23/05/2017 Salt water (e.g. seawater or brine) can be electrolysed using an electric current to produce chlorine and other useful products: Chlorine gas (Cl2) – useful for making bleach and plastics Hydrogen gas (H2) – useful as a potential fuel source or manufacture of HCl Sodium chloride solution (salt water) NaCl(aq) Positive electrode Negative electrode Sodium hydroxide (NaOH(aq)) – useful for making soaps and manufacture of paper Half equations for this process 23/05/2017 NaCl(aq) contains Na+, OH-, Cl- and H+ ions Chlorine gas (Cl2) Hydrogen gas (H2) Sodium chloride solution (salt water) Sodium hydroxide (made out of ions that haven’t discharged) NaCl(aq) At the anode: 2Cl- - 2e- Cl2 Oxidation Positive electrode (anode) Negative electrode (cathode) At the cathode: 2H+ + 2e- H2 Reduction Using Sodium Chloride in Industry 23/05/2017 A source of sodium hydroxide Products from it can be used to make bleach and PVC A source of chlorine Industrial uses for salt Hydrogen is used to make margarine Chlorine can sterilise water