Honors Chemistry
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
Unit 10 complete 2016-2017
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
85 Q.2 Pure water has a low electricity conductivity because A. it
... (1) They give the same colour change when the same quantity to universal indicator is added. (2) They react with marble chips at the same rate when the initial temperatures are the same. (3) They require the same number of moles of sodium hydroxide for complete neutralization. A. (1) only B. (3) onl ...
... (1) They give the same colour change when the same quantity to universal indicator is added. (2) They react with marble chips at the same rate when the initial temperatures are the same. (3) They require the same number of moles of sodium hydroxide for complete neutralization. A. (1) only B. (3) onl ...
Multiple-choice questions : 1. Which of the following solutions
... Each question below consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table ...
... Each question below consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table ...
Mole-Volume Conversion Assignment
... Deciding which reactants are the limiting reagents and the reactants in excess when given masses of both reactants: 1. Write the balanced chemical equation for the chemical reaction 2. Determine how many moles of each reactant you have. 3. Use the stoichiometric ratio to determine which reactant it ...
... Deciding which reactants are the limiting reagents and the reactants in excess when given masses of both reactants: 1. Write the balanced chemical equation for the chemical reaction 2. Determine how many moles of each reactant you have. 3. Use the stoichiometric ratio to determine which reactant it ...
Chemistry SAM
... through the glass the silver ions are reduced to silver atoms which cluster together and block the transmittance of light resulting in the darkening of the glass. The chloride ions are oxidised to chlorine atoms. ...
... through the glass the silver ions are reduced to silver atoms which cluster together and block the transmittance of light resulting in the darkening of the glass. The chloride ions are oxidised to chlorine atoms. ...
the pdf of this lesson!
... Pacific Ocean. It has a very interesting property of forming gels in the presence of calcium ions. Now you can make some terrific, colorific wormy gel things by connecting, or bonding, alginate with calcium ions. Recommended for ages 5 and up with adult supervision. This Kit Contains: 1 liter 0.8 % ...
... Pacific Ocean. It has a very interesting property of forming gels in the presence of calcium ions. Now you can make some terrific, colorific wormy gel things by connecting, or bonding, alginate with calcium ions. Recommended for ages 5 and up with adult supervision. This Kit Contains: 1 liter 0.8 % ...
Penetration of Synthetic Corticosteroids into Human
... tate (1%) penetrates through the cornea in significantly greater quantities than the other ophthalmic preparations analysed.6 Our pre liminary results for tluorometholone show that it reached the lowest peak concentration in aqueous humour of any of the five steroid preparations studied so far. How ...
... tate (1%) penetrates through the cornea in significantly greater quantities than the other ophthalmic preparations analysed.6 Our pre liminary results for tluorometholone show that it reached the lowest peak concentration in aqueous humour of any of the five steroid preparations studied so far. How ...
answer ch6 - Mr Khaled Nasr
... The volumes of gases involved in a reaction and the gases produced exist in fixed ratios. The law which states that equal volumes of gases under identical conditions of pressure and temperature contain equal numbers of particles. (6) A mass per unit volume of a substance. (7) A type of chemical anal ...
... The volumes of gases involved in a reaction and the gases produced exist in fixed ratios. The law which states that equal volumes of gases under identical conditions of pressure and temperature contain equal numbers of particles. (6) A mass per unit volume of a substance. (7) A type of chemical anal ...
Mole-Volume Conversion Assignment
... Ex. For a reaction between vinegar & baking soda (acetic acid and sodium bicarbonate): Note: A 5.0% solution of vinegar means there are 5.0 g of acetic acid for every 100mL of solution. So if we use 50mL of vinegar that means we are using 2.5g of acetic acid. What volume of carbon dioxide will be pr ...
... Ex. For a reaction between vinegar & baking soda (acetic acid and sodium bicarbonate): Note: A 5.0% solution of vinegar means there are 5.0 g of acetic acid for every 100mL of solution. So if we use 50mL of vinegar that means we are using 2.5g of acetic acid. What volume of carbon dioxide will be pr ...
Unit 7 Homework and Lab Packet
... 3. Some household drain cleaners consist of a mixture of granules of sodium hydroxide and powdered aluminum. When dissolved in water, the sodium hydroxide reacts with the aluminum to produce hydrogen gas: 2 Al + 2 NaOH + 6 H2O 2 NaAl(OH)4 + 3 H2 The sodium hydroxide helps dissolve grease, and the ...
... 3. Some household drain cleaners consist of a mixture of granules of sodium hydroxide and powdered aluminum. When dissolved in water, the sodium hydroxide reacts with the aluminum to produce hydrogen gas: 2 Al + 2 NaOH + 6 H2O 2 NaAl(OH)4 + 3 H2 The sodium hydroxide helps dissolve grease, and the ...
A Few Things You Might Want To Know
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
Acids ,Bases and Salts
... The more the dissociation the higher the yield of ions and the greater the electrical conductivity of the solution. A compound that conducts electricity in an electrolyte and thus a compound showing high electrical conductivity is a strong electrolyte while a compound showing low electrical conducti ...
... The more the dissociation the higher the yield of ions and the greater the electrical conductivity of the solution. A compound that conducts electricity in an electrolyte and thus a compound showing high electrical conductivity is a strong electrolyte while a compound showing low electrical conducti ...
Chemistry Tests Questions
... 30. State one physical difference between silicon dioxide and carbon dioxide. 31. Why did Mendeleev put silicon and carbon in the same vertical group? 32. Which reacts most readily with water, lithium or sodium? 33. Do copper, silver and gold react with water? 34. On which side of the periodic table ...
... 30. State one physical difference between silicon dioxide and carbon dioxide. 31. Why did Mendeleev put silicon and carbon in the same vertical group? 32. Which reacts most readily with water, lithium or sodium? 33. Do copper, silver and gold react with water? 34. On which side of the periodic table ...
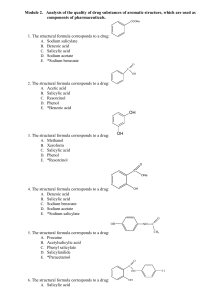
Module 2. Drug substances of aromatic structure
... A. Methyl orange B. Tropeolin 00 C. Methyl red D. Phenolphthalein E. *Solution of starch 81. The chemical name of xeroform: A. Oxybenzene B. Dihydroxybenzene C. 3-Methyl-5-methylphenol D. 5-Methyl-2-(methylethyl)phenol E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol i ...
... A. Methyl orange B. Tropeolin 00 C. Methyl red D. Phenolphthalein E. *Solution of starch 81. The chemical name of xeroform: A. Oxybenzene B. Dihydroxybenzene C. 3-Methyl-5-methylphenol D. 5-Methyl-2-(methylethyl)phenol E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol i ...
Questions
... Write an equation, including the state symbols, for the reaction between solid lithium chloride and concentrated sulphuric acid. ...
... Write an equation, including the state symbols, for the reaction between solid lithium chloride and concentrated sulphuric acid. ...
National 5 - Deans Community High School
... "Handwarmers" are intended for use by people who spend time in cold conditions. They consist of an outer packet made of strong plastic plus metal foil. The packet contains colourless crystals and a weak inner bag which is full of a grey powder. When the packet is rubbed between the hands, the inner ...
... "Handwarmers" are intended for use by people who spend time in cold conditions. They consist of an outer packet made of strong plastic plus metal foil. The packet contains colourless crystals and a weak inner bag which is full of a grey powder. When the packet is rubbed between the hands, the inner ...
Summer Study Assignment – Honors Chem 2/AP Chemistry
... must be obtained in the diet is methionine. What is the percentage of carbon, nitrogen, and sulfur in this amino acid if the formula of methionine is CH3SCH2CH2CHNH2COOH? 105. Ammonia is produced by the reaction of nitrogen and hydrogen according to this balanced equation: N2 (g) + 3 H2 (g) 2 NH3 ...
... must be obtained in the diet is methionine. What is the percentage of carbon, nitrogen, and sulfur in this amino acid if the formula of methionine is CH3SCH2CH2CHNH2COOH? 105. Ammonia is produced by the reaction of nitrogen and hydrogen according to this balanced equation: N2 (g) + 3 H2 (g) 2 NH3 ...
s-BLOCK ELEMENTS - einstein classes
... Mg forms a protective layer of oxide, so despite its favorable reduction potential, it does not react readily unless the oxide layer is removed by amalgamating with mercury. In the formation of the oxide film it resembles aluminium. ...
... Mg forms a protective layer of oxide, so despite its favorable reduction potential, it does not react readily unless the oxide layer is removed by amalgamating with mercury. In the formation of the oxide film it resembles aluminium. ...
Experimental skills and abilities
... 1 The evaporation process should be done very slowly. This is because sugar can easily char as it solidifies around the sides of the evaporating basin during the evaporating process. Also the crystallisation will require a lot longer for crystals to form from the concentrated solution and may need ...
... 1 The evaporation process should be done very slowly. This is because sugar can easily char as it solidifies around the sides of the evaporating basin during the evaporating process. Also the crystallisation will require a lot longer for crystals to form from the concentrated solution and may need ...
Chapter 8 - Inorganic carbon chemistry
... the UK annually, mainly by the Solvay process. Sodium carbonate is used for glass manufacture, brine treatment and water purification, as well as in the manufacture of detergents and textiles (Figure 8.12). ...
... the UK annually, mainly by the Solvay process. Sodium carbonate is used for glass manufacture, brine treatment and water purification, as well as in the manufacture of detergents and textiles (Figure 8.12). ...
Chemistry of METALS
... (i)has a pungent irritating smell that causes headache to human beings. (ii)bleaches any wet substance. (iii)dissolves water to form both hydrochloric acid and chloric(I)acid Both cause marine pollution and stomach upsets. 2.Sodium metal rapidly react with traces of water to form alkaline Sodium hyd ...
... (i)has a pungent irritating smell that causes headache to human beings. (ii)bleaches any wet substance. (iii)dissolves water to form both hydrochloric acid and chloric(I)acid Both cause marine pollution and stomach upsets. 2.Sodium metal rapidly react with traces of water to form alkaline Sodium hyd ...
1. A pharmacy analyst supervises the state of a refractometer. For its
... For this purpose he potassium permanganate and boil for 5 min; the solution remains faintly pink. brings some amount of the sample material to the boiling point, adds 0,02 M solution of potassium permanganate and diluted sulfuric acid. After 5 minutes of boiling the pink colour of the produced solut ...
... For this purpose he potassium permanganate and boil for 5 min; the solution remains faintly pink. brings some amount of the sample material to the boiling point, adds 0,02 M solution of potassium permanganate and diluted sulfuric acid. After 5 minutes of boiling the pink colour of the produced solut ...
Sodium bicarbonate

Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate) is a chemical compound with the formula NaHCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.It is among the food additives encoded by European Union, identified by the initials E 500.Since it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, and bicarbonate of soda. The word saleratus, from Latin sal æratus meaning aerated salt, was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.