CHEM 1405 Practice Exam #2
... A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) ...
... A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) ...
Unit C3, C3.1
... However, other chemists did not accept Newlands’ ideas. It was not until much later that his contribution to the development of the modern periodic table was recognised. Reproduced courtesy of the library and information centre Royal Society of Chemistry ...
... However, other chemists did not accept Newlands’ ideas. It was not until much later that his contribution to the development of the modern periodic table was recognised. Reproduced courtesy of the library and information centre Royal Society of Chemistry ...
reviewTWO
... How many moles of oxygen are needed to react with 0.1 mole of CH4 How many moles of CO2 are produced from 0.1 moles of CH4 How many moles of water are produced from 0.1 moles of CH4 How many moles of carbon dioxide will be produced by 0.1 mole of oxygen gas? How many moles of oxygen gas will react c ...
... How many moles of oxygen are needed to react with 0.1 mole of CH4 How many moles of CO2 are produced from 0.1 moles of CH4 How many moles of water are produced from 0.1 moles of CH4 How many moles of carbon dioxide will be produced by 0.1 mole of oxygen gas? How many moles of oxygen gas will react c ...
CHEMISTRY 101 Name Mock Final Exam Spring 2014 Signature Dr
... 27. Consider the reaction represented by the following unbalanced chemical equation NH3(g) + Cl2(g) → NH4Cl(s) + NCl3(g) What mass of NH4Cl can be produced from 10.0 g of NH3 and an excess of Cl2? a) 160.5 g b) 53.5 g c) 35.3 g d) 23.6 g e) None of these --------------------------------------------- ...
... 27. Consider the reaction represented by the following unbalanced chemical equation NH3(g) + Cl2(g) → NH4Cl(s) + NCl3(g) What mass of NH4Cl can be produced from 10.0 g of NH3 and an excess of Cl2? a) 160.5 g b) 53.5 g c) 35.3 g d) 23.6 g e) None of these --------------------------------------------- ...
Slide 1
... NOTE THAT THE NaClO OXIDISES IODIDE (I-) TO IODINE (I2) So as well as any possible yellow precipitate, you will also see the typical reddish-brown colour of iodine solution being formed during the reaction. Note also, that sodium chlorate(I) solution is alkaline and contains a sufficietly high [OH-] ...
... NOTE THAT THE NaClO OXIDISES IODIDE (I-) TO IODINE (I2) So as well as any possible yellow precipitate, you will also see the typical reddish-brown colour of iodine solution being formed during the reaction. Note also, that sodium chlorate(I) solution is alkaline and contains a sufficietly high [OH-] ...
10th CBSE {SA - 1} Revision Pack Booklet - 3
... black. However, when hydrogen is passed over the hot black substance so formed, it regains its original colour. Based on the given information, answer the following questions (a) Name the metal initially taken in the powder form. (b) What type of chemical reaction takes place in each of the two give ...
... black. However, when hydrogen is passed over the hot black substance so formed, it regains its original colour. Based on the given information, answer the following questions (a) Name the metal initially taken in the powder form. (b) What type of chemical reaction takes place in each of the two give ...
balancing chemical equations worksheet
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
2011-2012 Paper 1
... 6. Chlorine has a relative atomic mass of 35.5 and has two isotopes with relative isotopic masses of 35 and 37. Which of the following statements about chlorine are CORRECT? (1) The isotopes have same atomic number. (2) It contains the two isotopes, chlorine-35 and chlorine-37, in a ratio of 1:3. (3 ...
... 6. Chlorine has a relative atomic mass of 35.5 and has two isotopes with relative isotopic masses of 35 and 37. Which of the following statements about chlorine are CORRECT? (1) The isotopes have same atomic number. (2) It contains the two isotopes, chlorine-35 and chlorine-37, in a ratio of 1:3. (3 ...
Preparation of spherical DDNP study Liu off on a journey
... Conjugate salts of these compounds will be mixed to serious crystallization DDNP Impact of product purity. Therefore, diazo vessel, with water After the ammonia salts and polymorphs control agent dispersed, should first feed solution with pH Hydrochloric acid transferred close to neutral, and then a ...
... Conjugate salts of these compounds will be mixed to serious crystallization DDNP Impact of product purity. Therefore, diazo vessel, with water After the ammonia salts and polymorphs control agent dispersed, should first feed solution with pH Hydrochloric acid transferred close to neutral, and then a ...
GoLYTELY full Prescribing Information
... less frequently. These adverse reactions are transient and usually subside rapidly. Isolated cases of urticaria, rhinorrhea, dermatitis and (rarely) anaphylactic reaction have been reported which may represent allergic reactions. Published literature contains isolated reports of serious adverse reac ...
... less frequently. These adverse reactions are transient and usually subside rapidly. Isolated cases of urticaria, rhinorrhea, dermatitis and (rarely) anaphylactic reaction have been reported which may represent allergic reactions. Published literature contains isolated reports of serious adverse reac ...
Chemistry@YIA – additional information
... There are 3 basic problems making the jump: The first is making sure there are no gaps in your knowledge from GCSE. That is the main purpose of this pack. Second is the quantity of material that you have to cover and sorting out what’s important. It’s useful to identify patterns that you can then ‘h ...
... There are 3 basic problems making the jump: The first is making sure there are no gaps in your knowledge from GCSE. That is the main purpose of this pack. Second is the quantity of material that you have to cover and sorting out what’s important. It’s useful to identify patterns that you can then ‘h ...
AP CHEMISTRY SUMMER 2016
... a. F2 b. Cl2 c. C d. NaCl e. KF f. CO2 g. H2 h. Ag i. Rust (Fe2O3) j. MgO k. O2 l. I2 m.CO n. K2CO3 ...
... a. F2 b. Cl2 c. C d. NaCl e. KF f. CO2 g. H2 h. Ag i. Rust (Fe2O3) j. MgO k. O2 l. I2 m.CO n. K2CO3 ...
CHAPTER 6: Earth science
... colourless solution of sodium chloride in a test tube. (a) A white precipitate forms. Define the term ‘precipitate’. An insoluble solid forms when two solutions are mixed. ...
... colourless solution of sodium chloride in a test tube. (a) A white precipitate forms. Define the term ‘precipitate’. An insoluble solid forms when two solutions are mixed. ...
Chemistry Exam Review 2
... 1) 30g of solute is dissolved in 100g of water. Calculate its % (m/m) concentration. 2) Calculate the mass of solute that would be in 65g of a saturated solution, if its solubility is 35% (m/m). 3) 0.045 g of insecticide is found in a 1.7 kg sample. Calculate the concentration in ppm of insecticide ...
... 1) 30g of solute is dissolved in 100g of water. Calculate its % (m/m) concentration. 2) Calculate the mass of solute that would be in 65g of a saturated solution, if its solubility is 35% (m/m). 3) 0.045 g of insecticide is found in a 1.7 kg sample. Calculate the concentration in ppm of insecticide ...
Name ______ Write formulas for the reactants and predicted
... Solid ammonium carbonate is added to a saturated solution of barium hydroxide. (NH4)2CO3 + Ba2+ + 2 OH- ...
... Solid ammonium carbonate is added to a saturated solution of barium hydroxide. (NH4)2CO3 + Ba2+ + 2 OH- ...
Extraction lecture - UCLA Chemistry and Biochemistry
... • If the correct solvent was used for extraction, 2-3 extractions are usually sufficient to isolate the majority of the target compound • Unless large amounts of material are transferred from one phase to the other, the solvent/solution volume that should be used for extraction should not exceed 10- ...
... • If the correct solvent was used for extraction, 2-3 extractions are usually sufficient to isolate the majority of the target compound • Unless large amounts of material are transferred from one phase to the other, the solvent/solution volume that should be used for extraction should not exceed 10- ...
Lecture 11 - U of L Class Index
... The Group 2 elements are called alkaline earths. The ‘‘earth’’ part of the group name is left over from the days of medieval alchemy. To alchemists, any solid substance that did not melt and was not changed by fire into another substance was called an ‘‘earth.’’ Various compounds of Group 1 and 2 el ...
... The Group 2 elements are called alkaline earths. The ‘‘earth’’ part of the group name is left over from the days of medieval alchemy. To alchemists, any solid substance that did not melt and was not changed by fire into another substance was called an ‘‘earth.’’ Various compounds of Group 1 and 2 el ...
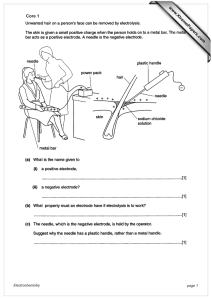
Core 1 www.XtremePapers.com Electrochemistry page 1
... does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock ...
... does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock ...
Combined
... 1. (a) Sodium hydroxide solution reacts with carbon dioxide gas [1] in air to form sodium carbonate: 2NaOH(aq) + CO2(g) Na2CO3(aq) + H2O(l) [1] The sodium carbonate formed reacts with dilute hydrochloric acid [1] to give colourless bubbles of carbon dioxide gas: Na2CO3(aq) + 2HCl(aq) 2NaCl(aq) + ...
... 1. (a) Sodium hydroxide solution reacts with carbon dioxide gas [1] in air to form sodium carbonate: 2NaOH(aq) + CO2(g) Na2CO3(aq) + H2O(l) [1] The sodium carbonate formed reacts with dilute hydrochloric acid [1] to give colourless bubbles of carbon dioxide gas: Na2CO3(aq) + 2HCl(aq) 2NaCl(aq) + ...
AP Chemistry Summer Assignment
... a. Kg b. Liter c. m3 d. mm e. kg/m3 f. Joule g. atm h. cal i. Torr j. g/ml 4. Most laboratory experiments are performed at room temperature at 65˚C. Express this temperature in: a. ˚F b. K 5. A cylinder rod formed from silicon is 46.0 cm long and has a mass of 3.00 kg. The density of silicon is 2.33 ...
... a. Kg b. Liter c. m3 d. mm e. kg/m3 f. Joule g. atm h. cal i. Torr j. g/ml 4. Most laboratory experiments are performed at room temperature at 65˚C. Express this temperature in: a. ˚F b. K 5. A cylinder rod formed from silicon is 46.0 cm long and has a mass of 3.00 kg. The density of silicon is 2.33 ...
Notes
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble substance formed by the reaction of ...
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble substance formed by the reaction of ...
Chemical Reactions
... to the left, because the reactant side has greater number of moles than does the product side. The system tries to counteract the decrease in partial pressure of gas molecules by shifting to the side that exerts greater pressure. Similarly, if we were to increase pressure by decreasing volume, the e ...
... to the left, because the reactant side has greater number of moles than does the product side. The system tries to counteract the decrease in partial pressure of gas molecules by shifting to the side that exerts greater pressure. Similarly, if we were to increase pressure by decreasing volume, the e ...
AP_chemistry_Summer_Assignment_2014
... B. K 5. A cylinder rod formed from silicon is 46.0 cm long and has a mass of 3.00 kg. The density of silicon is 2.33 g/cm3. What is the diameter of the cylinder? (the volume of cylinder is given by ∏ r2h, where r is the radius and h is the length) 6. How many significant figures are in each of the f ...
... B. K 5. A cylinder rod formed from silicon is 46.0 cm long and has a mass of 3.00 kg. The density of silicon is 2.33 g/cm3. What is the diameter of the cylinder? (the volume of cylinder is given by ∏ r2h, where r is the radius and h is the length) 6. How many significant figures are in each of the f ...
C4C5C6
... number (same number of protons) but a different number of neutrons so it has a different relative ...
... number (same number of protons) but a different number of neutrons so it has a different relative ...
Tests for functional groups
... Both Fehling’s and Benedict’s solutions contain complexed copper(II) ions in an alkaline solution. ...
... Both Fehling’s and Benedict’s solutions contain complexed copper(II) ions in an alkaline solution. ...
Sodium bicarbonate

Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate) is a chemical compound with the formula NaHCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs.It is among the food additives encoded by European Union, identified by the initials E 500.Since it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, and bicarbonate of soda. The word saleratus, from Latin sal æratus meaning aerated salt, was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.