* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Core 1 www.XtremePapers.com Electrochemistry page 1
Survey
Document related concepts
Nanofluidic circuitry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Water splitting wikipedia , lookup
Geochemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Acid–base reaction wikipedia , lookup
Gaseous detection device wikipedia , lookup
Sodium hypochlorite wikipedia , lookup
Sodium bicarbonate wikipedia , lookup
Sodium hydroxide wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
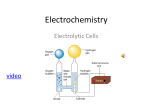
w w ap eP m e tr .X w Core 1 om .c s er Electrochemistry page 1 Core 1 Electrochemistry page 2 Core 2 Electrochemistry page 3 Alternative to Practical 1 Electrochemistry page 4 Extension 1 Electrochemistry page 5 Extension 2 Fig. 1 Electrochemistry page 6 Extension 2 Fig. 2 Electrochemistry page 7 Core 1 a(i) anode (ii) cathode b conducts electricity c does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock d(i) NaCl (ii) substance dissolved in liquid / contains dissolved substance (iii) hydrochloric acid sodium hydroxide / sodium carbonate / sodium bicarbonate (iv) add acid to the alkali until neutral / use titration evaporate off water / boil off water / leave to crystallise e hydrogen / H2 Electrochemistry page 1 Core 2 (i) lead sulphate (ii) oxygen has been added to it Electrochemistry page 2 Alternative to Practical 1 - a B – Cl attracted b Na / cation / positive ion c sodium chlorine d bubbles / silvery metal / green yellow gas + Electrochemistry page 3 Extension 1 i reducing 2+ germanium or Ge 2+ or Ge - 2e 3+ iron or Fe 3+ or Fe ii loses / donates electrons 4+ Ge gains electrons + e 2+ Fe sodium hydroxide or aqueous ammonia iron (III) salt brown precipitate iron (II) salt green precipitate (other possible reagents include iodide, thiocyanate, hexacyanoferrates, bromine, zinc, potassium manganate (VII) Electrochemistry page 4 Extension 2 a three of these points 2- Cu + 2e Cu - 2e Cu 2- Cu ions removed at cathode ions formed at anode b(i) copper formed or iron dissolves zinc not displaced or iron does not react with zinc ions (i) blue precipitate of copper hydroxide white precipitate of zinc hydroxide c(i) produces electrical energy or voltage or current from chemical energy or chemical reactions or two different electrodes in electrolyte (ii) from magnesium to iron through external circuit Electrochemistry page 5