Document
... As highlighted in the Preface, the descriptions for media referenced in Chapters <61> and <62> of the recently harmonized United States Pharmacopeia have been updated. Specifically, the section on “User Quality Control” contains the information required to verify that these media were tested accordi ...
... As highlighted in the Preface, the descriptions for media referenced in Chapters <61> and <62> of the recently harmonized United States Pharmacopeia have been updated. Specifically, the section on “User Quality Control” contains the information required to verify that these media were tested accordi ...
Chapter 12
... reactant needed to completely react with the limiting reactant, we can subtract that amount from the given amount to find the amount of excess. Can we find the amount of excess potassium in the previous problem? ...
... reactant needed to completely react with the limiting reactant, we can subtract that amount from the given amount to find the amount of excess. Can we find the amount of excess potassium in the previous problem? ...
Laboratory Works and Home Tasks in General Chemistry
... Thus, titer has the units of g/mL. Normality (CN). Normality is defined as the number of moles of the equivalent of a solute (neq) in 1 liter of a solution; that is, CN = neq (solute) / V (solution). Thus, normality has the units of mol/L or N. In chemical reactions the molar ratio of reacting subst ...
... Thus, titer has the units of g/mL. Normality (CN). Normality is defined as the number of moles of the equivalent of a solute (neq) in 1 liter of a solution; that is, CN = neq (solute) / V (solution). Thus, normality has the units of mol/L or N. In chemical reactions the molar ratio of reacting subst ...
Cookies and Chemistry…Huh!?!?
... How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
... How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
Chapter+12
... How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
... How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
"Cyano Compounds, Inorganic," in: Ullmann`s Encyclopedia of
... from the aqueous solution in a rectifier and condensed. The end product is highly pure and has a water content of less than 0.5 %. The aqueous absorber solution, containing traces of HCN, is cooled and fed back to the absorption tower. The residual gases, H2, CO, and N2, can be used for heating or m ...
... from the aqueous solution in a rectifier and condensed. The end product is highly pure and has a water content of less than 0.5 %. The aqueous absorber solution, containing traces of HCN, is cooled and fed back to the absorption tower. The residual gases, H2, CO, and N2, can be used for heating or m ...
APPROACHES TO CARBOHYDRATE-BASED CHEMICAL LIBRARIES: THE
... these potential benefits, the search for superior new leads is sufficient to justify an investment in combinatorial chemistry. Nevertheless, this stated purpose is artificially limiting and presents a somewhat myopic view of the potential of combinatorial chemistry. The methodical process of refini ...
... these potential benefits, the search for superior new leads is sufficient to justify an investment in combinatorial chemistry. Nevertheless, this stated purpose is artificially limiting and presents a somewhat myopic view of the potential of combinatorial chemistry. The methodical process of refini ...
File
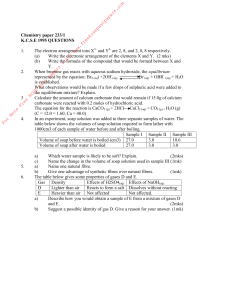
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
Grade XII Unit 1 - Ethiopian Ministry of Education
... Solution of liquids in liquids Ethanol mixes with water but oil does not. Why? Solubility is a measure of how much solute will dissolve in a solvent at a specific temperature. Do you know the principle “like dissolves like”? The “like dissolves like” principle is helpful in predicting the solubility ...
... Solution of liquids in liquids Ethanol mixes with water but oil does not. Why? Solubility is a measure of how much solute will dissolve in a solvent at a specific temperature. Do you know the principle “like dissolves like”? The “like dissolves like” principle is helpful in predicting the solubility ...
МЕТОДИЧЕСКИЕ УКАЗАНИЯ СТУДЕНТАМ
... 1. What volume (in mL) of 40% H3PO4 solution (ρ=1,25 g/cm3) is necessary to prepare 400mL of 0,25N of phosphoric acid solution (ρ=1g/cm3)? Calculate the mole fraction of H3PO4 in the obtained solution. 2. How many grams of Na2CO3·10H2O are necessary to prepare 100mL of 10% Na2CO3 solution (ρ=1,12 g/ ...
... 1. What volume (in mL) of 40% H3PO4 solution (ρ=1,25 g/cm3) is necessary to prepare 400mL of 0,25N of phosphoric acid solution (ρ=1g/cm3)? Calculate the mole fraction of H3PO4 in the obtained solution. 2. How many grams of Na2CO3·10H2O are necessary to prepare 100mL of 10% Na2CO3 solution (ρ=1,12 g/ ...
General and Inorganic Chemistry – Laboratory Techniques
... When the atomic theory developed to the point where it was possible to write specific formulae for the various oxides and their binary compounds, names reflecting composition more or less accurately then became common. As a number of inorganic compounds rapidly grew, the essential pattern of nomencl ...
... When the atomic theory developed to the point where it was possible to write specific formulae for the various oxides and their binary compounds, names reflecting composition more or less accurately then became common. As a number of inorganic compounds rapidly grew, the essential pattern of nomencl ...
PVP-Iodine Brochure
... The microbiocidal action of PVP-Iodine, as discussed earlier, is related to the non-complexed, freely mobile elemental iodine, I2, the active form of which is polarized by water and hence can be considered to be H2OI+ in its final state. This activated iodine reacts in electrophilic reactions with e ...
... The microbiocidal action of PVP-Iodine, as discussed earlier, is related to the non-complexed, freely mobile elemental iodine, I2, the active form of which is polarized by water and hence can be considered to be H2OI+ in its final state. This activated iodine reacts in electrophilic reactions with e ...
General and Inorganic Chemistry
... ammonium chloride solutions of unknown concentrations .................................. 39 4.4. III.4.4 Measurement of temperature ....................................................................... 40 4.5. III.4.5 Warming and boiling ............................................................ ...
... ammonium chloride solutions of unknown concentrations .................................. 39 4.4. III.4.4 Measurement of temperature ....................................................................... 40 4.5. III.4.5 Warming and boiling ............................................................ ...
Topic 6 Section C
... Chlorine bleach must be stored in cool places. This is because hypochlorite is unstable and it gradually decomposes into chloride and oxygen. 2OCl-(aq) 2Cl-(aq) + O2(g) This decomposition takes place faster if the temperature is higher. ...
... Chlorine bleach must be stored in cool places. This is because hypochlorite is unstable and it gradually decomposes into chloride and oxygen. 2OCl-(aq) 2Cl-(aq) + O2(g) This decomposition takes place faster if the temperature is higher. ...
engineering chemistry
... 'atomos', meaning 'unable to be cut'. The original meaning of atom was the smallest, indivisible form of a chemical particle. Now we know how to divide atoms into sub-atomic particles, the definition of an atom includes the concept that the particle must retain its chemical properties. ATOM An Atom ...
... 'atomos', meaning 'unable to be cut'. The original meaning of atom was the smallest, indivisible form of a chemical particle. Now we know how to divide atoms into sub-atomic particles, the definition of an atom includes the concept that the particle must retain its chemical properties. ATOM An Atom ...
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
44. Find рН of formic acid solution with mass percent ω=5
... of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentration of the equivalent 0,1 mol/L. 18. Calculate molar concentration of 4% glucose solut ...
... of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentration of the equivalent 0,1 mol/L. 18. Calculate molar concentration of 4% glucose solut ...
Stoichiometry
... Instead of calling them recipes, we call them chemical equations Furthermore, instead of using cups and teaspoons, we use moles Lastly, instead of eggs, butter, sugar, etc. we use chemical compounds as ingredients ...
... Instead of calling them recipes, we call them chemical equations Furthermore, instead of using cups and teaspoons, we use moles Lastly, instead of eggs, butter, sugar, etc. we use chemical compounds as ingredients ...
Chemistry Club Demos - 10-8-15
... the gas outlet and pilot light. Then cut/drill two medium (1/2”) holes in the side of the can, roughly 1” from the rim of the opening and opposite each other; these act as air-intake vents. Cover all three holes with adhesive tape such that they can be opened quickly. Finally, use pliers to com ...
... the gas outlet and pilot light. Then cut/drill two medium (1/2”) holes in the side of the can, roughly 1” from the rim of the opening and opposite each other; these act as air-intake vents. Cover all three holes with adhesive tape such that they can be opened quickly. Finally, use pliers to com ...
kcse chemistry questions
... State what would be observed when dilute hydrochloric acid is added to the products formed when a mixture of iron fillings and sulphur? (1mk) Describe how the following reagents can be used to prepare lead sulphate solid potassium sulphate, solid lead carbonate, dilute nitric acid and distilled wate ...
... State what would be observed when dilute hydrochloric acid is added to the products formed when a mixture of iron fillings and sulphur? (1mk) Describe how the following reagents can be used to prepare lead sulphate solid potassium sulphate, solid lead carbonate, dilute nitric acid and distilled wate ...
the chemical and physical properties of condensed
... of preparing these polyphosphate salts are not satisfactory. Some work has been done toward relating the size of a cation to the type of phosphate which can be crystallized from a melt2' 8O More work is needed in this area. The existing phase diagrams seem to indicate that compounds containing an ev ...
... of preparing these polyphosphate salts are not satisfactory. Some work has been done toward relating the size of a cation to the type of phosphate which can be crystallized from a melt2' 8O More work is needed in this area. The existing phase diagrams seem to indicate that compounds containing an ev ...
Unit 10 complete 2016-2017
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
Honors Chemistry
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
... White phosphorus (P4) is used in military missiles because it ignites (reacts with oxygen) spontaneously in air to produce tetraphosphorus decoxide. How many grams of P4 will react with 25.0 grams of oxygen? ...
CFREP - QTR Review Presentation Template
... Summary • Spraying phage into a stomacher bag holding the inoculated sample may have resulted in an inadequate dose of phage coming in contact to the inoculated spots on the food. • Due to the passive movement of phage in food high concentrations are needed to ensure contact with the bacteria ...
... Summary • Spraying phage into a stomacher bag holding the inoculated sample may have resulted in an inadequate dose of phage coming in contact to the inoculated spots on the food. • Due to the passive movement of phage in food high concentrations are needed to ensure contact with the bacteria ...
Sodium hypochlorite

Sodium hypochlorite is a chemical compound with the formula NaClO. It is composed of a sodium cation (Na+) and a hypochlorite anion (ClO−); it may also be viewed as the sodium salt of hypochlorous acid. When dissolved in water it is commonly known as bleach, or liquid bleach. Sodium hypochlorite is practically and chemically distinct from chlorine. Sodium hypochlorite is frequently used as a disinfectant or a bleaching agent.