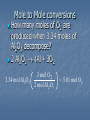* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cookies and Chemistry…Huh!?!?
Acid dissociation constant wikipedia , lookup
Kinetic resolution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Acid–base reaction wikipedia , lookup
Electrochemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Click chemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Process chemistry wikipedia , lookup
Water splitting wikipedia , lookup
Sodium hypochlorite wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
George S. Hammond wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Implicit solvation wikipedia , lookup
Rate equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Geometrical frustration wikipedia , lookup
Cookies and Chemistry…Huh!?!? Just like chocolate chip cookies have recipes, chemists have recipes as well Instead of calling them recipes, we call them reaction equations Furthermore, instead of using cups and teaspoons, we use moles Lastly, instead of eggs, butter, sugar, etc. we use chemical compounds as ingredients Chemistry Recipes Looking at a reaction tells us how much of something you need to react with something else to get a product (like the cookie recipe) Be sure you have a balanced reaction before you start! Example: 2 Na + Cl2 2 NaCl This reaction tells us that by mixing 2 moles of sodium with 1 mole of chlorine we will get 2 moles of sodium chloride What if we wanted 4 moles of NaCl? 10 moles? 50 moles? 2H2 + O2 2H2O Two molecules of hydrogen and one molecule of oxygen form two molecules of water. 2 Al2O3 Al + 3O2 2 formula units Al2O3 form 4 atoms Al and 3 molecules O2 Now try this: 2Na + 2H2O 2NaOH + H2 Practice Write the balanced reaction for hydrogen gas reacting with oxygen gas. 2 H2 + O 2 2 H2 O How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? Mole Ratios These mole ratios can be used to calculate the moles of one chemical from the given amount of a different chemical Example: How many moles of chlorine are needed to react with 5 moles of sodium (without any sodium left over)? 2 Na + Cl2 2 NaCl 5 moles Na 1 mol Cl2 2 mol Na = 2.5 moles Cl2 Mole-Mole Conversions How many moles of sodium chloride will be produced if you react 2.6 moles of chlorine gas with an excess (more than you need) of sodium metal? Mole-Mole Conversions 2.6 moles Cl2 2 mol NaCl 1 mol Cl2 5.2 mol NaCl Mole to Mole conversions 2 Al2O3 Al + 3O2 each time we use 2 moles of Al2O3 we will also make 3 moles of O2 2 moles Al2O3 3 mole O2 or 3 mole O2 2 moles Al2O3 These are the two possible conversion factors Mole to Mole conversions How many moles of O2 are produced when 3.34 moles of Al2O3 decompose? 2 Al2O3 Al + 3O2 3.34 mol Al2O3 3 mol O2 2 mol Al2O3 = 5.01 mol O2 Mole-Mass Conversions Most of the time in chemistry, the amounts are given in grams instead of moles We still go through moles and use the mole ratio, but now we also use molar mass to get to grams Example: How many grams of chlorine are required to react completely with 5.00 moles of sodium to produce sodium chloride? 2 Na + Cl2 2 NaCl 5.00 moles Na 1 mol Cl2 2 mol Na 70.90g Cl2 1 mol Cl2 = 177g Cl2 Practice Calculate the mass in grams of Iodine required to react completely with 0.50 moles of aluminum. Practice: Mole Mass Conversions Balanced Equation: 2 Al + 3 I2 ---> 2 AlI3 Stoichiometry: .5 mol Al 3 mol I2 2 mol Al 252g I 1 mol I2 Mass-Mole We can also start with mass and convert to moles of product or another reactant We use molar mass and the mole ratio to get to moles of the compound of interest Calculate the number of moles of ethane (C2H6) needed to produce 10.0 g of water 2 C2H6 + 7 O2 4 CO2 + 6 H20 10.0 g H2O 1 mol H2O 2 mol C2H6 = 0.185 18.0 g H2O 6 mol H20 mol C2H6 Mass-Mass Conversions Most often we are given a starting mass and want to find out the mass of a product we will get (called theoretical yield) or how much of another reactant we need to completely react with it (no leftover ingredients!) Now we must go from grams to moles, mole ratio, and back to grams of compound we are interested in Mass-Mass Conversion Ex. Calculate how many grams of ammonia are produced when you react 2.00g of nitrogen with excess hydrogen. N2 + 3 H2 2 NH3 2.00g N2 1 mol N2 2 mol NH3 17.06g NH3 28.02g N2 1 mol N2 = 2.4 g NH3 1 mol NH3 Mass-Mass Practice Calculate how many grams of oxygen are required to make 10.0 g of aluminum oxide Balanced Equation: 3 O2 + 4 Al -> 2 Al2O3 Mole Ratio Needed: 2 mole Al2O3 to 3 mol O2 Stoichiometry: 10.0g 3 mol O2 2 mol Al2O3 32 g O2 1 mol O2 Practice: 2C2H2 + 5 O2 4CO2 + 2 H2O • If 3.84 moles of C2H2 are burned, how many moles of O2 are needed? (9.6 mol) •How many moles of C2H2 are needed to produce 8.95 mole of H2O? (8.95 mol) •If 2.47 moles of C2H2 are burned, how many moles of CO2 are formed? (4.94 mol) Mass-Mass Problem: 6.50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4 Al + 3 O2 2Al2O3 6.50 g Al 1 mol Al 2 mol Al2O3 101.96 g Al2O3 26.98 g Al 4 mol Al 1 mol Al2O3 = ? g Al2O3 (6.50 x 2 x 101.96) ÷ (26.98 x 4) = 12.3 g Al2O3 Another example: If 10.1 g of Fe are added to a solution of Copper (II) Sulfate, how much solid copper would form? 2Fe + 3CuSO4 Fe2(SO4)3 + 3Cu Answer = 17.2 g Cu Volume-Volume Calculations: How many liters of CH4 at STP are required to completely react with 17.5 L of O2 ? CH4 + 2O2 CO2 + 2H2O 1 mol O2 1 mol CH4 22.4 L CH4 17.5 L O2 22.4 L O2 2 mol O2 1 mol CH4 = 8.75 L CH4 Notice anything concerning these two steps? Solution Stoichiometry 50.0 mL of 6.0 M H2SO4 (battery acid) were spilled and solid NaHCO3 (baking soda) is to be used to neutralize the acid. How many grams of NaHCO3 must be used? H2SO4(aq) + 2NaHCO3 2H2O(l) + Na2SO4(aq) + 2CO2(g) 50.0 mL ?g 6.0 M = 6.0 mol L H2SO4 50.0 mL 6.0 mol H 2SO 4 1000mL H 2SO 4 NaHCO3 NaHCO3 84.0 g 2 mol = 50.4 g NaHCO3 mol 1 mol NaHCO3 H2SO4 gram to gram conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3.45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6HCl(aq) 2AlCl3(aq) + ? grams 3.45 g 3 H2(g) Units match 3.45 g Al mol Al 27.0 g Al 2 mol AlCl 3 133.3 g AlCl 3 mol AlCl 3 2 mol Al Now We must Now use Let’s the always use work molar thethe convert molar mass problem. ratio. to toconvert moles. to grams. = 17.0 g AlCl3 Solution Stoichiometry What volume of 0.40 M HCl solution is needed to completely neutralize 47.1 mL of 0.75 M Ba(OH)2? 2HCl(aq) + Ba(OH)2(aq) 0.40 M 47.1 mL 0.75 M ? mL Ba(OH)2 47.1 mL 0.75mol Ba(OH)2 2H2O(l) + BaCl2 Units match HCl 2 mol 1000 mL Ba(OH)2 1 mol Ba(OH)2 HCl 1000 mL 0.40 mol HCl = 176 mL HCl Solution Stochiometry Problem: A chemist performed a titration to standardize a barium hydroxide solution. If it took 23.28 mL of 0.135 M hydrochloric acid to neutralize 25.00 mL of the barium hydroxide solution, what was the concentration of the barium hydroxide solution in moles per liter (M)? 2 1 2 2O(l) + ____BaCl 1 ____HCl(aq) + ____Ba(OH) 2(aq) ____H 2(aq) 25.00 mL 23.28 mL Units match on top! ? mol 0.135 mol L L 23.28 mL HCl 25.00 x 10-3 L Ba(OH)2 0.135 mol HCl l mol Ba(OH)2 = 0.0629 mol Ba(OH)2 1000 mL HCl 2 mol HCl L Ba(OH) 2 Units Already Match on Bottom! Limiting Reactant: Cookies 1 cup butter 1/2 cup white sugar 1 cup packed brown sugar 1 teaspoon vanilla extract 2 eggs 2 1/2 cups all-purpose flour 1 teaspoon baking soda 1 teaspoon salt 2 cups semisweet chocolate chips Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? Limiting Reactant Most of the time in chemistry we have more of one reactant than we need to completely use up other reactant. That reactant is said to be in excess (there is too much). The other reactant limits how much product we get. Once it runs out, the reaction s. This is called the limiting reactant. Limiting Reactant To find the correct answer, we have to try all of the reactants. We have to calculate how much of a product we can get from each of the reactants to determine which reactant is the limiting one. The lower amount of a product is the correct answer. The reactant that makes the least amount of product is the limiting reactant. Once you determine the limiting reactant, you should ALWAYS start with it! Be sure to pick a product! You can’t compare to see which is greater and which is lower unless the product is the same! Limiting Reactant: Example 10.0g of aluminum reacts with 35.0 grams of chlorine gas to produce aluminum chloride. Which reactant is limiting, which is in excess, and how much product is produced? 2 Al + 3 Cl2 2 AlCl3 Start with Al: 10.0 g Al 1 mol Al 27.0 g Al 2 mol AlCl3 133.5 g AlCl3 2 mol Al 1 mol AlCl3 = 49.4g AlCl3 Now Cl2: 35.0g Cl2 1 mol Cl2 71.0 g Cl2 2 mol AlCl3 133.5 g AlCl3 3 mol Cl2 1 mol AlCl3 = 43.9g AlCl3 LR Example Continued We get 49.4g of aluminum chloride from the given amount of aluminum, but only 43.9g of aluminum chloride from the given amount of chlorine. Therefore, chlorine is the limiting reactant. Once the 35.0g of chlorine is used up, the reaction comes to a complete . If 10.6 g of copper reacts with 3.83 g sulfur, how many grams of product (copper Cu is Limiting Reagent (I) sulfide) will be formed? 2Cu + S Cu2S 1 mol Cu2S 159.16 g Cu2S 1 mol Cu 10.6 g Cu 63.55g Cu 2 mol Cu 1 mol Cu2S = 13.3 g Cu2S 1 mol S 3.83 g S 32.06g S 1 mol Cu2S 159.16 g Cu2S 1 mol S 1 mol Cu2S = 19.0 g Cu2S Limiting Reactant Practice 15.0 g of potassium reacts with 15.0 g of iodine. Calculate which reactant is limiting and how much product is made. Finding the Amount of Excess By calculating the amount of the excess reactant needed to completely react with the limiting reactant, we can subtract that amount from the given amount to find the amount of excess. Can we find the amount of excess potassium in the previous problem? Finding Excess Practice 15.0 g of potassium reacts with 15.0 g of iodine. 2 K + I2 2 KI We found that Iodine is the limiting reactant, and 19.6 g of potassium iodide are produced. We must now find the amound of K actually used. 15.0 g I2 1 mol I2 2 mol K 39.1 g K 254 g I2 1 mol I2 1 mol K = 4.62 g K USED! 15.0 g K – 4.62 g K = 10.38 g K EXCESS Given amount of excess reactant Amount of excess reactant actually used Note that we started with the limiting reactant! Once you determine the LR, you should only start with it! Limiting/Excess/ Reactant and Theoretical Yield Problems : Potassium superoxide, KO2, is used in rebreathing gas masks to generate oxygen. 4KO2(s) + 2H2O(l) 4KOH(s) + 3O2(g) a. How many moles of O2 can be produced from 0.15 mol KO2 and 0.10 mol H2O? b. Determine the limiting reactant. 4KO2(s) + 2H2O(l) 4KOH(s) + 3O2(g) 0.15.15 mol ? moles 0.10 mol Based on: 0.15 mol KO2 KO2 3mol O 2 4mol KO 2 Based on: 0.10 mol H2O 3mol O 2 H2 O 2mol H O 2 = 0.1125 mol O2 = 0.150 mol O2 Limiting Reactant: Recap 1. 2. 3. 4. 5. 6. 7. You can recognize a limiting reactant problem because there is MORE THAN ONE GIVEN AMOUNT. Convert ALL of the reactants to the SAME product (pick any product you choose.) The lowest answer is the correct answer. The reactant that gave you the lowest answer is the LIMITING REACTANT. The other reactant(s) are in EXCESS. To find the amount of excess, subtract the amount used from the given amount. If you have to find more than one product, be sure to start with the limiting reactant. You don’t have to determine which is the LR over and over again! Percent Yield To determine percentage yield, you need two pieces of information: 1) Theoretical yield = Comes from the stoichiometry 2) Actual yield = Comes from experimental data Percent Yield = Actual Yield Theoretical Yield 100%