* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Update on New Indications for BMT
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
List of types of proteins wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
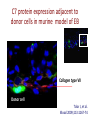
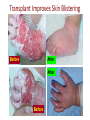
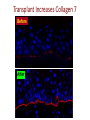
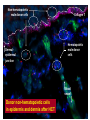
New Indications for BMT: Extracellular Matrix Disorder Dystrophic Epidermolysis Bullosa from Jakub Tolar, MD, PhD Blood and Marrow Transplantation University of Minnesota Collagen Type VII = Anchoring Fibrils Anchoring Fibrils = VELCRO To determine whether systemically administered hematopietic cells can correct collagen type VII deficiency in recessive dystrophic epidermolysis bullosa. C7 protein expression adjacent to donor cells in murine model of EB Collagen type VII Donor cell Tolar J, et al. Blood 2009;113:1167-74 Clinical Trial for RDEB Clinical Trial. Eligibility 1. Dx of RDEB 2. 0-25 years 3. Adequate donor 4. Acceptable organ function 5. No SCC Transplant CYCLOPHOSPHAMIDE BUSULPHAN -9 -8 -7 -6 FLUDARABINE -5 -4 Skin Evaluations at days 0, 28, 60, 100, -3 -2 -1 0 Days MYCOPHENYLATE 6 months, 1 year, and 2 and years 2 years 1.Photographs of skin and oral mucosa CYCLOSPORINE 2.Collagen type VII immunofluorescence 3.Electron microscopy (evaluated by blinded reviewers) Transplant Improves Skin Blistering Before After After Before Transplant Increases Collagen 7 Before After Non-hematopoietic male donor cells Dermalepidermal junction Collagen 7 Hematopoietic male donor cells Blood vessel Donor non-hematopoietic cells in epidermis and dermis after HCT Summary Adoptive transfer of bone marrow and cord blood cells results in functional correction of recessive dystrophic epidermolysis bullosa. Clinical Trial. Clinical Trial II for RDEB Eligibility 1. Dx of RDEB 2. 0-25 years 3. Adequate donor 4. Acceptable organ function 5. No SCC Transplant HSC+MSC CYCLOPHOSPHAMIDE BUSULPHAN -9 -8 -7 -6 FLUDARABINE -5 -4 Skin Evaluations at days 0, 28, 60, 100, -3 -2 -1 0 Days MYCOPHENYLATE 6 months, 1 year, and 2 and years 2 years 1.Photographs of skin and oral mucosa CYCLOSPORINE 2.Collagen type VII immunofluorescence 3.Electron microscopy (evaluated by blinded reviewers)