* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mutations in genes
E. coli long-term evolution experiment wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Genome evolution wikipedia , lookup
Community fingerprinting wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Genetic code wikipedia , lookup
Non-coding DNA wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
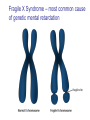
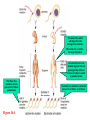
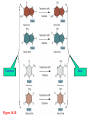
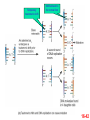
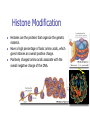
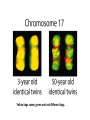
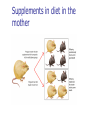
Genetics: Gene Mutations and DNA Repair INTRODUCTION The term mutation refers to a heritable change in the genetic material Mutations provide allelic variations On the positive side, mutations are the foundation for evolutionary change E.g. Light skin in high latitude human populations On the negative side, mutations are the cause of many diseases E.g. Hemophilia DNA Maintenance Mutation rate are extremely low 1 mutation out of 109 nucleotides per generation CONSEQUENCES OF MUTATIONS Mutations can be divided into three main types 1. Chromosome mutations 2. Genome mutations Changes in chromosome number 3. Single-gene mutations Changes in chromosome structure Relatively small changes in DNA structure that occur within a particular gene Type 3 will be discussed in this set of lecture notes Gene Mutations Change the DNA Sequence A point mutation is a change in a single base pair It involves a base substitution 5’ AACGCTAGATC 3’ 3’ TTGCGATCTAG 5’ 5’ AACGCGAGATC 3’ 3’ TTGCGCTCTAG 5’ A transition is a change of a pyrimidine (C, T) to another pyrimidine or a purine (A, G) to another purine A transversion is a change of a pyrimidine to a purine or vice versa Transitions are more common than transversions Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. © Phototake/Alamy Normal red blood cells © Phototake/Alamy 10 μm Sickled red blood cells 10 μm (a) Micrographs of red blood cells NORMAL : NH2 – VALINE – HISTIDINE – LEUCINE – THREONINE – PROLINE – GLUTAMIC ACID – GLUTAMIC ACID... SICKLE : NH2 – VALINE – HISTIDINE – LEUCINE – THREONINE – PROLINE – VALINE– GLUTAMIC ACID... CELL (b) A comparison of the amino acid sequence between normal b-globin and sickle-cell b-globin 7 Mutations may also involve the addition or deletion of short sequences of DNA 5’ AACGCTAGATC 3’ 3’ TTGCGATCTAG 5’ 5’ AACGCTC 3’ 3’ TTGCGAG 5’ Deletion of four base pairs 5’ AACGCTAGATC 3’ 3’ TTGCGATCTAG 5’ 5’ AACAGTCGCTAGATC 3’ 3’ TTGTCAGCGATCTAG 5’ Addition of four base pairs Gene Mutations Can Alter the Coding Sequence Within a Gene Mutations in the coding sequence of a structural gene can have various effects on the polypeptide Silent mutations are those base substitutions that do not alter the amino acid sequence of the polypeptide Due to the degeneracy of the genetic code E.g. AUU to AUC still codes for Ile Missense mutations are those base substitutions in which an amino acid change does occur Example: Sickle-cell anemia If the substituted amino acid does not affect protein function (as measured by phenotype), the mutation is said to be neutral Nonsense mutations are those base substitutions that change a normal codon to a termination codon Frameshift mutations involve the addition or deletion of nucleotides in multiples of one or two This shifts the reading frame so that a completely different amino acid sequence occurs downstream from the mutation Gene Mutations and Their Effects on Genotype and Phenotype In a natural population, the wild-type is the most common genotype (may be encoded by a dominant or recessive allele) A forward mutation changes the wild-type genotype into some new variation If it is beneficial, it may move evolution forward Otherwise, it will be probably eliminated from a population A reverse mutation has the opposite effect It is also termed a reversion (changes mutant back to wild-type) Mutations can also be described based on their effects on the wild-type phenotype When a mutation alters an organism’s phenotypic characteristics, it is said to be a variant Variants are often characterized by their differential ability to survive Deleterious mutations decrease the chances of survival The most extreme are lethal mutations E.g. Homozygous polydactyly in cats Beneficial mutations enhance the survival or reproductive success of an organism Some mutations are called conditional mutants They affect the phenotype only under a defined set of conditions (such as temp affecting wild type and mutant bacteria) A second mutation will sometimes affect the phenotypic expression of another These second-site mutations are called suppressor mutations or simply suppressors Suppressor mutations are classified into two types Intragenic suppressors The second mutant site is within the same gene as the first mutation Intergenic suppressors The second mutant site is in a different gene from the first mutation It may make it revert to normal (wild-type) or add more mutations Researchers showed that mutations caused by either a single base insertion (+) or a single base deletion (-) could be “suppressed” or restored by a second mutation of the opposite sign, as long as the two mutations occurred in the same vicinity of the gene. Gene Mutations in Non-coding Sequences These mutations can still affect gene expression A mutation, may alter the sequence within a promoter Up promoter mutations make the promoter more like the consensus sequence Down promoter mutations make the promoter less like the consensus sequence They may increase the rate of transcription They may decrease the rate of transcription Probably responsible for most differences between closely-related organisms (e.g. humans and chimps) A mutation can also alter splice junctions in eukaryotes (3'-UTR) is the section of messenger RNA (mRNA) that immediately follows the translation termination codon. Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 16-13 Mutations Due to Trinucleotide Repeats Several human genetic diseases are caused by an unusual form of mutation called trinucleotide repeat expansion (TNRE) The term refers to the phenomenon that a sequence of 3 nucleotides can increase from one generation to the next These diseases include Huntington disease (HD) Fragile X syndrome (FRAXA) Fragile X Syndrome – most common cause of genetic mental retardation Certain regions of the chromosome contain trinucleotide sequences repeated in tandem In normal individuals, these sequences are transmitted from parent to offspring without mutation However, in persons with TRNE disorders, the length of a trinucleotide repeat increases above a certain critical size It also becomes prone to frequent expansion This phenomenon is shown here with the trinucleotide repeat CAG CAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAG n = 11 CAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAGCAG n = 18 Genetic Anticipation Analysis of the rare families in which TNRE diseases occur has revealed that expansion of the triplet repeats is linked to something called genetic anticipation when a disease’s symptoms appear earlier and more severely in each successive generation. In some cases, the expansion is within the coding sequence of the gene Typically the trinucleotide expansion is CAG (glutamine) Therefore, the encoded protein will contain long tracks of glutamine This causes the proteins to aggregate with each other This aggregation is correlated with the progression of the disease In other cases, the expansions are located in noncoding regions of genes These expansions are hypothesized to cause abnormal changes in RNA structure Thereby producing disease symptoms Changes in Chromosome Structure Can Affect Gene Expression A chromosomal rearrangement may affect a gene because the break occurred in the gene itself A gene may be left intact, but its expression may be altered because of its new location This is termed a position effect Movement may be next to a regulatory sequence or into into a heterochromatic region and now expressed. Regulatory sequences are often bidirectional Mutations Can Occur in Germ-Line or Somatic Cells Geneticists classify the animal cells into two types Germ-line cells Somatic cells Cells that give rise to gametes such as eggs and sperm All other cells Germ-line mutations are those that occur directly in a sperm or egg cell, or in one of their precursor Somatic mutations are those that occur directly in a body cell, or in one of its precursor cells The size of the patch will depend on the timing of the mutation The earlier the mutation, the larger the patch An individual who has somatic regions that are genotypically different from each other is called a genetic mosaic Therefore, the mutation can be passed on to future generations Figure 16.4 Therefore, the mutation cannot be passed on to future generations OCCURRENCE AND CAUSES OF MUTATION Mutations can occur spontaneously or be induced Spontaneous mutations Result from abnormalities in cellular/biological processes Induced mutations Caused by environmental agents Agents that are known to alter DNA structure are termed mutagens Errors in DNA replication, for example These can be chemical or physical agents Refer to Table 16.4 Mutation Rates and Frequencies The mutation frequency for a gene is the number of mutant genes divided by the total number of genes in a population. Ex – if 1 million bacteria were plated and 10 were mutant, the mutation frequency would be I in 100,000 or 10-5 Mutation frequency also depends on Timing of the mutation Likelihood that the mutation will be passed on to future generations Mutation rate In genetics, the mutation rate is a measure of the rate at which various types of mutations occur over time. Mutation rates are typically given for a specific class of mutation, for instance point mutations, small or large scale insertions or deletions. Spontaneous Mutations Are Random Events Are mutations spontaneous occurrences or causally related to environmental conditions? This is a question that biologists have asked themselves for a long time Jean Baptiste Lamarck Proposed that physiological events (e.g. use and disuse) determine whether traits are passed along to offspring Charles Darwin Proposed that genetic variation occurs by chance Natural selection results in better-adapted organisms Random Mutations Can Give an Organism a Survival Advantage Directed mutation theory Selected conditions could promote the formation of specific mutations allowing the organism to survive This was consistent with Lamarck’s viewpoint Random mutation theory – most widely accepted Environmental factors simply select for the survival of those individuals that happen to possess beneficial mutations This was consistent with Darwin’s viewpoint Causes of Spontaneous Mutations Spontaneous mutations can arise by three types of chemical changes 1. Depurination 2. Deamination 3. Tautomeric shift The most common Causes of Spontaneous Mutations Depurination involves the removal of a purine (guanine or adenine) from the DNA The covalent bond between deoxyribose and a purine base is somewhat unstable It occasionally undergoes a spontaneous reaction with water that releases the base from the sugar Fortunately, these can be repaired However, if the repair system fails, a mutation may result during subsequent rounds of DNA replication Three out of four (A, T and G) are the incorrect nucleotide There’s a 75% chance of a mutation Spontaneous depurination Deamination involves the removal of an amino group from the cytosine base The other bases are not readily deaminated DNA repair enzymes can recognize uracil as an inappropriate base in DNA and remove it However, if the repair system fails, a C-G to A-T mutation will result during subsequent rounds of DNA replication Deamination of 5-methyl cytosine can also occur Thymine is a normal constituent of DNA This poses a problem for repair enzymes They cannot determine which of the two bases on the two DNA strands is the incorrect base For this reason, methylated cytosine bases tend to create hot spots for mutation A tautomeric shift involves a temporary change in base structure These rare forms promote AC and GT base pairs For a tautomeric shift to cause a mutation it must occur immediately prior to DNA replication Common Figure 16.10 Rare Temporary tautomeric shift Shifted back to its normal fom 16-42 Types of Mutagens An enormous array of agents can act as mutagens to permanently alter the structure of DNA The public is concerned about mutagens for two main reasons: 1. Somatic mutagens are often involved in the development of human cancers 2. Germ-line mutations may have harmful effects in future generations Mutagenic agents are usually classified as chemical or physical mutagens Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 16-53 Nonionizing radiation Includes UV light Has less energy Cannot penetrate deeply into biological molecules Causes the formation of cross-linked thymine dimers Thymine dimers may cause mutations when that DNA strand is replicated Damage from X-rays Causes base deletions Single nicks in DNA molecule Cross-linking Chromosomal breaks DNA Repair All species have a variety of DNA repair systems to avoid the harmful effects of mutations. Excision repair recognizes and removes a damaged base or damaged segments of DNA. Base mismatch repair recognizes a base mismatch and removes a segment of the DNA strand with the incorrect base. Mismatch Repair DNA polymerase replaces missing or damaged bases. Excision Repair Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. 57 Xeroderma pigmentosum, or XP, is an autosomal recessive genetic disorder of DNA repair in which the ability to repair damage caused by ultraviolet (UV) light is deficient. The absorption of the highenergy light leads to the formation of pyrimidine dimers photoproducts. In a healthy, normal human being, the damage is first excised by endonucleases. DNA polymerase then repairs the missing sequence, and ligase "seals" the transaction. This process is known as nucleotide excision repair. Symptoms include: Severe sunburn when exposed to only small amounts of sunlight. These often occur during a child's first exposure to sunlight. Development of many freckles at an early age Rough-surfaced growths and skin cancers Eyes that are painfully sensitive to the sun and may easily become irritated, bloodshot and clouded Blistering or freckling on minimum sun exposure Spider Veins Limited growth of hair on chest and legs Scaly skin Dry skin Irregular dark spots on the skin Corneal ulcerations Mutant Hemoglobin Lab EPIGENETICS http://www.pbs.org/wgbh/nova/body/epigenet ics.html https://www.youtube.com/watch?v=9AfB sTAQ8zs&edufilter=ITEV1GgfY7rUuGxSyy 0SA Epigenetics Epigenetic Research The number of publications in the field has increased dramatically in the last 10 years. • Genetic Engineering and Biotechnology News Feb 1, 2013 (Vol. 33, No. 3) Epigenetic Effects The effects of epigenetics have been known for many years. Lyon Hypothesis from 1961. Renamed the Lyon Law in 2011. Inactive X chromosome is heavily epigenetically modified. Another Familiar Epigenetic Case: Genomic imprinting Ex - Chromosome 15 imprinting Prader-Willi Syndrome Angelman Syndrome This is, the offspring cell distinguishes between the maternally and the paternally inherited allele by means of the different methylation patterns. Thus, the phenotype of the offspring depends on the sex of the parent who has inherited an imprinted allele due to promoter inhibition of one allele by DNA-methylation. Imprinting is not the cause of these syndromes but is responsible for the unique presentation of these diseases. Genomic imprinting is probably responsible for hereditary defects caused by in-vitro fertilization and cloning of mammals (e.g. "Dolly" born with a vast burden of false epigenetic information due to sole maternal imprinting). Children born after conception with the help of "Assisted Reproductive Technologies" (ARTs) showed significant lower birth weight, a higher incidence of juvenile cancer and an increased frequency of Beckwith-Wiedemann and Angelman syndrome, respectively (Schieve et al., Obstet. Gynecol 2004; Gosden et al., Lancet 2003; Niemetz and Feinberg Am J Hum Genet 2004). Importantly, X-inactivation and genomic imprinting are normal processes. Much of the recent research has analyzed when the process of epigenetics is altered from normal. This has involved the study of changes within somatic cells in disease. It has also involved the study of changes within the germ cells (heritable epigenetics). Heritable Epigenetics Evidence suggests that environmental information could be propagated through meiosis. Överkalix Study Retrospective study conducted in Överkalix, Sweden. Divided population into three cohorts: Born 1890 • Born 1905 Born 1920 Assessed each cohort for access to food during slow growth period (SGP) of adolescence (8-10 girls, 9-12 boys). Överkalix Study Results When the father was exposed to a famine during his SGP, his offspring exhibited protection against cardiovascular causes of death. Paternal grandmother exposure to famine also showed a trend towards similar protection in grandchildren. If the paternal grandfather lived through a famine during his SGP it tended to protect grandchildren from diabetes. If the paternal grandfather had an abundance of food during their SGP, their grandchildren had a four-fold increased risk for death of diabetes mellitus. One mechanism to explain these results is transmission of epigenetic markers that were influenced by the environment of the parent. Effect on grandchildren suggests the markers are maintained through multiple generations. Lamarkism? Jean Batiste Lamark (1744-1829) Inheritance of acquired characteristics. Largely discounted with Darwin’s publication of Origin of Species and the rediscovery of work of Mendel. Recent work in epigenetics suggest Larmark may have been correct to some degree. Molecular Basis of Epigenetics Two primary mechanisms identified. Methylation of cytosine nucleotides in DNA Modification to histone proteins. Includes acetylation, methylation and phosphorylation A third proposed mechanism involves expression of small interfering RNAs (siRNA). Cytosine Methylation Methylation of cytosine occurs at CpG dinucleotides. Often located just upstream of genes (promoter regions). Associated with attenuation of expression of nearby genes. Histone Modification Histones are the proteins that organize the genetic material. Have a high percentage of basic amino acids, which gives histones an overall positive charge. Positively charged amino acids associate with the overall negative charge of the DNA. Histone Modification Most histone modification occurs on the extended tails of histone proteins. Modifications influence the association of histones with the DNA and patterns of gene expression. Best studied modification is histone acetylation. Epigenetic Errors Fragile X syndrome is most commonly caused by a CGG trinucleotide repeat expansion in the 5’ region of the FMR1 gene. Unaffected individuals have 6-50 CGG repeats. >200 CGG repeats is seen in individuals with fragile X. >200 CGG repeats is correlated with hypermethylation at CpG dinucleotides and silencing of the FMR1 gene. Epigenetics and Cancer DNA repair is a critical process to maintain genomic fidelity. Loss of DNA repair is thought to be a major contributor to the development of cancer. Epigenetic changes involving DNA repair genes are thought to be a major early step in cancer progression. ~13% of sporadic breast cancers and 5-30% of ovarian cancers present with hypermethylation of the BRCA1 gene. 40-90% of sporadic colorectal cancer has hypermethylation of the MGMT gene (O6methylguanine methyltransferase). Therapies Targeting Epigenetic Errors In contrast to mutations, epigenetic changes can be reversed. Are there therapies that influence epigenetic patterns? Vorinostat (trade name Zolinza) approved by FDA for cutaneous T cell lymphoma in 2006. Yes Vorinostat is a histone deacetylase inhibitor. ⬆ acetylation = ⬆gene expression. X Our Epigenome If epigenetic markers are dynamic and respond to environmental influences, do they change over time? Evidence suggests the answer is yes. Twin studies have been highly informative for this question. Yellow tags same; green and red different tags. Epigenomic Alterations If the epigenome changes as we age, what kinds of things can induce these changes? Very active area of current research. Some interesting findings: Fear conditioning induces changes in DNA methylation in rat brains. In rats, social deprivation during the 1st postnatal week triggers changes in DNA methylation across the gene that stimulates neuron development. Studies with cocaine exposure suggest that the drug induces acetylation of the BDNF gene histones that is transmittable to future male offspring. Smoking Nicotine causes epigenetic changes in mice that spur cocaine addiction What’s the News: Epidemiologists have long noticed that people with drug addictions often start out smoking cigarettes before moving on to harder stuff. Whether that’s because there’s something about cigarettes that makes people vulnerable to other drugs or because certain kinds of people are predisposed to addiction (or for some other reason entirely) is an open question, and the idea of so-called “gateway drugs” has been a controversial topic in addiction for years. Now, an elegant new study in mice has discovered a mechanism that could explain the gateway drug effect: nicotine actually changes the expression of genes linked to addiction. Read article below. http://blogs.discovermagazine.com/80beats/2011/11/05/nicotinecauses-epigenetic-changes-in-mice-that-spur-cocaine-addiction/ Supplements in diet in the mother Lick your rats http://learn.genetics.utah.edu/content/epigenetics/rats/