* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download structure_property
Rosetta@home wikipedia , lookup
List of types of proteins wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein design wikipedia , lookup
Implicit solvation wikipedia , lookup
Protein purification wikipedia , lookup
Western blot wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Structural alignment wikipedia , lookup
Homology modeling wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein domain wikipedia , lookup
Protein folding wikipedia , lookup
Circular dichroism wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Metalloprotein wikipedia , lookup
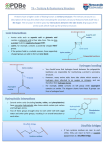
1 Protein Structure: protein architectures Park, Jong Hwa MRC-DUNN Hills Road Cambridge CB2 2XY England Bioinformatics in Biosophy : Next 02/06/2001 Protein Shapes In 1947, Max Perutz, MRC Laboratory of Molecular Biology in Cambridge Protein structure modelling (or determination) using X-ray diffraction. In 1950’s and 60’s, John Kendrew and Perutz solved the structures of Myoglobin and Haemoglobin. Since then many protein structures were solved in Cambridge and the researchers who were trained in Cambridge (many of them were americans). Kendrew and Perutz found out that : Structures are much more conserved than protein sequences in terms of residue variability. Modern Molecular biology has been dominated by Structures. It is because Structures are the most definite representation of proteins. http://www.techfak.uni-bielefeld.de/bcd/Curric/syllabi.html What makes protein architecture Protein structures need to achieve : 1) relatively Low energy conformation of individual residues(side groups) 2) hydrogen bonding by polar groups, including buried ones 3) formation of compact, well-packed structures (for most of them). For 1: Secondary structures are formed For 2: Secondary structures are formed For 3: Coils (turns) are used. The study of protein architecture is largely a description of the spatial assembly of helices and strands of sheet within the structure. Aminio Acid Side chains (R): The side chains confer important properties on a protein such as the ability to bind ligands and catalyse biochemical reactions. They also direct the folding of the nascent polypeptide and stabilise its final conformation. Certain colours are used conventionally to represent the different atom types found in proteins. red is used for oxygen, blue for nitrogen, white for carbon and yellow for sulphur. Hydrogen atoms cannot be located in most protein structures since they scatter X-rays too weakly. Alipatic amino acid side groups and proline, glycine Side Chain Carbon The side chain atoms of amino acids are named in the Greek alphabet according to this scheme. Side Chain Torsion Angle The side chain torsion angles are named chi1, chi2, chi3, etc., as shown below for lysine. Chi 1 Angle restriction The chi1 angle is subject to certain restrictions which arise from steric hindrance between the gamma side chain atom(s) and the main chain. The different conformations of the side chain as a function of chi1 are refered to as gauche(+), trans and gauche(-). These are indicated in the diagrams below in which the amino acid is viewed along the Cbeta-Calpha bond. Gauche - : looking top-down Trans form of Chi angle to C-alpha Gauche + is dominent • The most abundant conformation is gauche(+) in which the gamma side chain atom is opposite to the residue's main chain carbonyl group when viewed along the Cbeta-Calpha bond. • The second most abundant conformation is trans in which the side chain gamma atom is opposite the main chain nitrogen. • The least abundant conformation is gauche(-) which occurs when the side chain is opposite the hydrogen substituent on the Calpha atom. This conformation is unstable because the gamma atom is in close contact with the main chain CO and NH groups. The gauche(-) conformation is occasionally adopted by serine or threonine residues in a helix where the steric hindrance is offset by a hydrogen bond between the gamma oxygen atom and the main chain. • With most amino acids the gauche(+) and trans conformations are adopted with similar abundances although the gauche(+) conformation tends to dominate. Alpha Helix: N-H group in ith residue forms a hydrogen bond to the O=C group of residue i+4 Almost of all alpha helix is right handed. One residue to the next is about 100 degree 3D translation of 1.5 Angstrom in proteins. 3-10 helix: ith residue’s NH O=C of i+3th residue. Pi-helix ith => i+5th residue. Where the stability is from Toilet roll representation of alpha Distortions of alpha The majority of alpha-helices in globular proteins are curved or distorted somewhat compared with the standard Pauling-Corey model. These distortions arise from several factors including: 1. The packing of buried helices against other secondary structure elements in the core of the protein. Distortions 1. 2. 3. 4. 5. Proline residues induce distortions of around 20 degrees in the direction of the helix axis. This is because proline cannot form a regular alphahelix due to steric hindrance arising from its cyclic side chain, which also blocks the main chain N atom and chemically prevents it forming a hydrogen bond. Janet Thornton has shown that proline causes two H-bonds in the helix to be broken since the NH group of the following residue is also prevented from forming a good hydrogen bond. Helices containing proline are usually long perhaps because shorter helices would be destabilised by the presence of a proline residue too much. Proline occurs more commonly in extended regions of polypeptide. Solvent: Exposed helices are often bent away from the solvent region. This is because the exposed C=O groups tend to point towards solvent to maximise their H-bonding capacity, ie tend to form H-bonds to solvent as well as N-H groups. This gives rise to a bend in the helix axis. Solvent caused distortion of alpha http://pps98.man.poznan.pl/ppscore/section3/jonc/helix2.html 3-10 helices 1. 310-Helices. Strictly, these form a distinct class of helix 2. 3. but they are always short and frequently occur at the termini of regular alpha-helices. The name 310 arises because there are three residues per turn and ten atoms enclosed in a ring formed by each hydrogen bond (note the hydrogen atom is included in this count). There are main chain hydrogen bonds between residues separated by three residues along the chain (ie O(i) to N(i+3)). In this nomenclature the Pauling-Corey alphahelix is a 3.6(13)-helix. The dipoles of the 310-helix are not so well aligned as in the alpha-helix, ie it is a less stable structure and side chain packing is less favourable. The a-helix conformation has a particular stability for two main reasons. 1) Firstly the side chain groups are quite well separated. 2) Secondly, and most importantly, each peptide link is involved in two hydrogen bonds. (NH=> OC) The atoms involved are arranged linearly (figure 8) so that the hydrogen bonds are nearly at their maximum strength. The hydrogen bonds run down the length of the a-helix tube and lock the conformation in place. Alpha helix The structure repeats itself every 5.4 Angstroms along the helix axis, ie we say that the alpha-helix has a pitch of 5.4 Angstroms. Alpha-helices have 3.6 amino acid residues per turn, ie a helix 36 amino acids long would form 10 turns. Beta sheet: Parallel and anti-parallel Beta Topology Antiparallel beta-sheet Beta sheets are twisted Ramachandran plot In a polypeptide the main chain N-C.alpha and C.alpha-C bonds relatively are free to rotate. These rotations are represented by the torsion angles phi and psi, respectively. G N Ramachandran used computer models of small polypeptides to systematically vary phi and psi with the objective of finding stable conformations. For each conformation, the structure was examined for close contacts between atoms. Atoms were treated as hard spheres with dimensions corresponding to their van der Waals radii. Therefore, phi and psi angles which cause spheres to collide correspond to sterically disallowed conformations of the polypeptide backbone. Ramachandran Plot pic. Beta Bulge One important distortion of the pleated beta-sheet is known as the beta-bulge. These distortions arise whenever an amino acid is introduced in a beta-strand between residues which are forming the closely spaced hydrogen bonding pattern characteristic of beta-sheet. This disrupts the sheet which means that beta-bulges can only occur in an outer strand of the sheet. There are two main classes of beta-bulge known as as the classic and the G1 bulge. BetaBulge in Ramachandran plot BetaBulge a classic beta-bulge at position 156 of the superoxide dismutase from the tuberculosis mycobacterium. The rightmost of the three strands contains the bulge and the disruption to the sheet's hydrogen bonding pattern can be seen (dashed lines show Hbonds). Reverse Turns The structural elements that allow a sharp change of direction of the polypeptide chain are called reverse turns. Reverse turns are very abundant in globular proteins and generally occur at the surface of the molecule. It has been suggested that turn regions act as nucleation centres during protein folding. Reverse turns are divided into classes based on the type of secondary structure they link, on the number of residues in the turn and their phi and psi angles. The nomenclature can be confusing in that the term reverse turn and the terms for its subtypes are sometimes used interchangeably. Further confusion may arise from differing definitions. Gamma turns Reverse Turns Gammaturns include 3 consecutive residues and have a hydrogen bond from the main chain carbonyl oxygen O(i) to the main chain NH(i+2) group. Two types are distinguished. Betaturns include 4 consecutive residues and have a distance of less than 7Å between the C -atoms of residues i and i+3. Classically there is a hydrogen bond present between the main chain carbonyl oxygen O(i) and the main chain NH(i+3) group. Nine classes of Betaturn can be distinguished. Gamma Turn Beta Turns (type 1 and 2) Betaturns are divided into classes based on the phi and psi angles of the residues at positions i+1 and i+2. Types I and II shown in the figure below are the most common reverse turns, the essential difference between them being the orientation of the peptide bond between residues at (i+1) and (i+2). Type 1 and 2 , Beta Turns The torsion angles for the residues (i+1) and (i+2) in the two types of turn lie in distinct regions of the Ramachandran plot. Disulphide Bond Disulphide Bond Disulphide bonds or bridges are covalent bonds (ca. 2.08Å) and as such are part of the primary structure of the protein. However, they will also be involved in proper folding in three dimensions because they may link up non-consecutive parts of the polypeptide or two individual polypeptides. The geometry of disulphides can be described with 5 dihedral angles. Types of DS bond A thiol group which may form a covalent link with the thiol group of another cysteine. Disulphide bridges are sensitive to reducing agents which convert the two sulphur atoms back to their original -S-H form. Disulphide bridges are normally only present in extracellular proteins. References •Introduction to Protein Structure, Branden, C. and Tooze, J. (1991) Garland Publishing, New York •Proteins, Creighton, T.E. (1993) 2nd edition, W.H. Freeman & Co., New York •Principles of Protein Structure, Schulz, G.E. and Schirmer, R.H. (1979) Springer-Verlag, New York •Protein Structure - New Approaches to Disease and Therapy, Perutz, M. (1992) W.H. Freeman & Co., New York •Enzyme Structure and Mechanism, Fersht, A.R. (1976) 2nd ed., pub. W.H.Freeman & Co., New York •http://www.dcc.unicamp.br/~bio/ICMB.html