* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 6 and 7
Ancestral sequence reconstruction wikipedia , lookup
Self-assembling peptide wikipedia , lookup
Bottromycin wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Protein adsorption wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Metabolomics wikipedia , lookup
Gel electrophoresis wikipedia , lookup
Community fingerprinting wikipedia , lookup
Western blot wikipedia , lookup
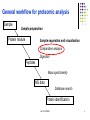
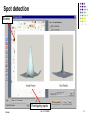
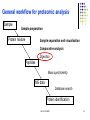
Lecture 6 Comparative analysis Oct 2011 SDMBT 1 General workflow for proteomic analysis Sample Sample preparation Protein mixture Sample separation and visualisation Comparative analysis Digestion Peptides Mass spectrometry MS data Database search Protein identification Oct 2011 SDMBT 2 Sequence of events for comparative analysis Scanning of image Image processing Spot Detection Gel Matching Data analysis Oct 2011 SDMBT 3 Scanning of image Convert ‘analog’ spots on gel into digital data High resolution images on densitometers/imaging systems For wet or dried gels that have been stained, X-ray films and blots Oct 2011 SDMBT (Biorad, Biosurplus.com, Institute of Arctic Biology) 4 Densitometry (UIC) (Proteomic Identification of 14-3-3ζ as an Adapter for IGF-1 and Akt/GSK-3β Signaling and Survival of Renal Mesangial Cells, Singh et al., Int J Biol Sci 2007; 3:27-39 ) Oct 2011 SDMBT 5 Densitometry Oct 2011 SDMBT 6 Image Processing Digital data converted into Gaussian curves. Algorithms used to smoothen curve, removing statistical noise Contrast enhancement to see better spots Background subtraction to remove meaningless changes in the background of the gel Oct 2011 SDMBT 7 Smoothing Gaussian curves Raw data curve #1 curve #2 Oct 2011 SDMBT (BARS,Statlib) (CBU Imaging Wiki) 8 Contrast enhancement (brneurosci.org) Oct 2011 SDMBT 9 Background subtraction (NIH Image) Oct 2011 SDMBT 10 Image Processing (Olympus) Oct 2011 SDMBT 11 Contrast enhancement (Olympus) Oct 2011 SDMBT 12 Smoothing (Olympus) Oct 2011 SDMBT 13 Background subtraction (Olympus) Oct 2011 SDMBT 14 Spot detection Automatic detection aided by manual input Need to adjust sensitivity Too little sensitivity = missed spots Too much sensitivity = false positives (Biorad) Oct 2011 SDMBT 15 Spot detection Streaks Overlapping spots (Biorad) Oct 2011 SDMBT 16 Gel Matching Compare identical spots on different gels Matching is seldom 100% due to variations in experimental techniques (staining, gel preparation) Use of landmarks to improve matching Most time-consuming step (Proteomics – from protein sequence to function, Pennington & Dunn [editors]) Oct 2011 SDMBT 17 Gel Matching Oct 2011 SDMBT (Biorad) 18 Manual spot matching Matched Unmatched (Biorad) Oct 2011 SDMBT 19 Data Analysis After matching, data are arranged into a table Subjected to normalisation to account for inconsistencies in staining and gel preparation Normalise by: •Total gel intensity •Total intensity of subset of spots (Proteomics – from protein sequence to function, Pennington & Dunn [editors]) Oct 2011 SDMBT 20 Data Analysis (Biorad) Oct 2011 SDMBT 21 e.g. with CyDye (GE Bioscience) Oct 2011 SDMBT 22 Oct 2011 SDMBT 23 Internal standard to make sure that abundance is normalised and variation Is due to biological variation rather than gel-to-gel variation Oct 2011 SDMBT 24 Lecture 7 In-gel digestion Oct 2011 SDMBT 25 General workflow for proteomic analysis Sample Sample preparation Protein mixture Sample separation and visualisation Comparative analysis Digestion Peptides Mass spectrometry MS data Database search Protein identification Oct 2011 SDMBT 26 Rationale for digestion of proteins Error is proportional to mass of the protein PTMs further complicate assignments based on mass Sensitivity of MS measurement increases with the use of smaller peptides (6-20 amino acids) Proteases are able to cut at specific amino acid residues Oct 2011 SDMBT 27 Trypsin Arginine or Lysine (ExPasy PeptideCutter) Proline Oct 2011 SDMBT 28 Chymotrypsin Leucine, Methionine and Histidine (minor) Tryptophan, Tyrosine and Phenylalanine (major) (ExPasy PeptideCutter) Proline Oct 2011 SDMBT 29 Peptide masses from tryptic digest Peptide Cutter (Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels, Shevchenko et al., Anal. Chem. 1996, 68, 850-858) Oct 2011 SDMBT 30 Typical protocol for in-gel digestion •Excision of Commassie stained spot(s) from gel(s) •Destaining with NH4HCO3 /acetonitrile •Reduction with DTT •Alkylation with IAA Oct 2011 SDMBT 31 Overview of in-gel digestion •Absorption of minimal amount of trypsin into gel (on ice) •Overnight incubation of trypsin at 37ºC •Extraction of peptides from gel with 5% formic acid in NH4HCO3 /acetonitrile, or trifluoroacetic acid •Clean up by ZipTips (removes ionic salts) Oct 2011 SDMBT 32