* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 11 Chemical Reactions
Nucleophilic acyl substitution wikipedia , lookup
Atomic theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
History of chemistry wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Asymmetric induction wikipedia , lookup
History of electrochemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Catalytic reforming wikipedia , lookup
Marcus theory wikipedia , lookup
Acid–base reaction wikipedia , lookup
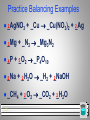
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Water splitting wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Click chemistry wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Rate equation wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Electrolysis of water wikipedia , lookup
George S. Hammond wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chapter 11 “Chemical Reactions” 1 Section 11.1 p. 321 Describing Chemical Reactions 2 All chemical reactions… 3 have two parts: 1. Reactants = substances you start with 2. Products = end with reactants turn into products Reactants Products - Page 321 Products Reactants 4 In a chem rxn Atoms not created or destroyed (Law of Conservation of Mass) rxn described in a: #1. sentence every item is a word Copper reacts with chlorine to form copper (II) chloride. #2. word equation some symbols used Copper + chlorine copper (II) chloride 5 Symbols in equations? – Text page 323 arrow (→) separates reactants from products (points to products) –Read as: “reacts to form” or yields plus sign = “and” (s) after formula = solid: Fe(s) (g) = gas: CO2(g) (l) = liquid: H2O(l) 6 Symbols used in equations (aq) after formula = dissolved in water, aqueous solution: NaCl(aq) is salt water solution used after product - indicates gas produced: H2↑ used after product - indicates solid produced: PbI2↓ 7 Symbols used in equations ■ double arrow indicates a reversible reaction (more later) heat ■ shows that , heat supplied to rxn Pt ■ indicates catalyst supplied (here, platinum is catalyst) 8 What is a catalyst? substance that speeds up rxn, w/o being changed or used up in rxn Enzymes - biological or protein catalysts in your body 9 #3. The Skeleton Equation Uses formulas and symbols to describe rxn –but doesn’t indicate how many; means they’re NOT balanced All chem equations are description of rxn 10 Write a skeleton equation for: 1. 2. 11 Solid iron (III) sulfide reacts with gaseous hydrogen chloride to form iron (III) chloride and hydrogen sulfide gas. Nitric acid dissolved in water reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate dissolved in water. Now, read these equations: Fe(s) + O2(g) Fe2O3(s) Cu(s) + AgNO3(aq) Ag(s) + Cu(NO3)2(aq) Pt NO2(g) 12 N2(g) + O2(g) #4. Balanced Chemical Equations Atoms can’t be created or destroyed in an ordinary reaction: –All atoms we start with we must end up with (balanced!) balanced equation has same # of each element on both sides of equation 13 Rules for balancing: 1) Assemble correct formulas for all reactants and products, using “+” and “→” 2) Count # of atoms of each type on both sides 3) Balance elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! (hint: I prefer to save O until the very last) 4) Double-Check make sure balanced 14 Never change subscript (only change coefficients) – changing subscript (formula) describes different chemical – H2O different than H2O2 Never put coefficient in middle of formula; only in front 2NaCl is okay, but Na2Cl is not. 15 Practice Balancing Examples _AgNO 2 3 _Mg 3 _P 4 + _N2 _Mg3N2 + _O 5 2 _P4O10 _Na 2 + _H 2 2 2O _H2 + _NaOH _CH4 16 + _Cu _Cu(NO3)2 + 2_Ag + _O 2 2 2O 2 _CO2 + _H Balancing Equations Balancing Chemical Reactions Mark Rosengarten – 8:21 17 Section 11.2 p. 330 Types of Chemical Reactions 18 Types of Reactions 5 major types. predict the products predict whether or not they will happen at all How? We recognize them by their reactants 19 #1 - Combination Reactions Combine = put together 2 substances combine to make one cmpd (also called “synthesis”) Ca + O2 CaO SO3 + H2O H2SO4 predict products, especially if reactants are 2 elements Mg3N2 (symbols, charges, cross) Mg + N2 _______ 20 Complete and balance: + Cl2 Fe + O2 (assume iron (II) oxide is the product) Al + O2 Ca first step…write correct formulas – you can still change subscripts at this point, but not while balancing! Then balance by changing just coefficients only Remember 21 #1 – Combination Reactions Additional Notes: a) Some nonmetal oxides react with H2O - produces acid: SO2 + H2O H2SO3 (how “acid rain” forms) b) Some metallic oxides react with H2O - produces base: CaO + H2O Ca(OH)2 22 #2 - Decomposition Reactions decompose = fall apart one reactant breaks apart into 2 or more elements or cmpds electricity NaCl Na + Cl2 CaCO3 CaO + CO2 Note: energy (heat, sunlight, electricity, etc.) usually required 23 #2 - Decomposition Reactions predict products if binary cmpd (made of 2 elements) –It breaks apart into the elements: electricity H2O HgO 24 #2 - Decomposition Reactions If cmpd has > 2 elements you must be given one of products –other product from the missing pieces NiCO3 CO2 + ___ H2CO3(aq) CO2 + ___ heat 25 #3 - Single Replacement Reactions One element replaces another (new dance partner) Reactants must be an element & cmpd Products will be a different element and different cmpd Na + KCl K + NaCl (Cations switched) F2 + LiCl LiF + Cl2 (Anions switched) 26 #3 Single Replacement Reactions Metals replace other metals (they can also replace H) K + AlN Zn + HCl Think of water as: HOH – Metals replace first H, then combines w/ hydroxide (OH). Na + HOH 27 #3 Single Replacement Reactions can even tell whether or not single replacement rxn will happen: –b/c some chemicals more “active” than others –More active replaces less active list – p. 333 - Activity Series of Metals Higher 28 on list replaces lower The “Activity Series” of Metals Higher activity Lower activity 29 Lithium Potassium Calcium Sodium Magnesium Aluminum Zinc Chromium Iron Nickel Lead Hydrogen Bismuth Copper Mercury Silver Platinum Gold 1) Metals can replace other metals, if they are above metal trying to replace (i.e. Zn will replace Pb) 2) Metals above H can replace H in acids. 3) Metals from Na upward can replace hydrogen in H2O The “Activity Series” of Halogens Higher Activity Fluorine Chlorine Bromine Iodine Halogens can replace other halogens in compounds, if they are above halogen they are replacing Lower Activity 2NaCl(s) + F2(g) MgCl2(s) + Br2(g) 30 2NaF ??? (s) + Cl2(g) ???Reaction! No #3 Single Replacement Reactions Practice: Fe + CuSO4 Pb + KCl Al + HCl 31 #4 - Double Replacement Reactions Two things replace each other. – Reactants must be two ionic compounds, in aqueous solution NaOH + FeCl3 – positive ions change place (dance partners) NaOH + FeCl3 Fe+3 OH- + Na+1 Cl-1 = NaOH + FeCl3 Fe(OH)3 + NaCl 32 #4 - Double Replacement Reactions Have certain “driving forces”, or reasons –only happens if one product: a) doesn’t dissolve in water & forms solid (a “precipitate”), or b) is gas that bubbles out, or c) is molecular compound (usually water) 33 Complete and balance: assume all of the following reactions actually take place: CaCl2 + NaOH CuCl2 + K2S KOH + Fe(NO3)3 (NH4)2SO4 + BaF2 34 How to recognize which type? Look at the reactants: E + E = Combination C = Decomposition E + C = Single replacement C + C = Double replacement 35 Practice Examples: + O2 H2O Zn + H2SO4 HgO KBr + Cl2 AgNO3 + NaCl Mg(OH)2 + H2SO3 H2 36 #5 – Combustion Reactions Combustion means “add oxygen” Normally, a cmpd composed of only C, H, (and maybe O) is reacted with oxygen – called “burning” Complete combustion, products are CO2 and H2O If incomplete, products are CO (or possibly just C) and H2O 37 Combustion Reaction Examples: C4H10 + O2 (assume complete) C4H10 + O2 (incomplete) C6H12O6 C8H8 38 + O2 (complete) + O2 (incomplete) SUMMARY: An equation... Describes a rxn Must be balanced (follows the Law of Conservation of Mass) only balance by changing coefficients special symbols to indicate physical state, catalyst or energy required, etc. 39 Reactions 5 major types We can tell what type they are by looking at reactants Single Replacement happens based on the Activity Series Double Replacement happens if one product is: 1) a precipitate (an insoluble solid), 2) water (a molecular compound), or 3) a gas 40 Section 11.3 p. 342 Reactions in Aqueous Solution NiCl2 Co(NO3)2 K2Cr2O7 41 K2CrO4 CuSO4 KMnO4 Net Ionic Equations Many reactions occur in water- that is, in aqueous solution When dissolved in water, many ionic cmpds “dissociate”, or separate, into cations & anions Now write ionic equation 42 Net Ionic Equations Example (needs to be a double replacement reaction) AgNO3 + NaCl AgCl + NaNO3 1. this is the full balanced equation 2. next, write it as ionic equation by splitting the cmpds into their ions: Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “precipitate” (Table 11.3 p. 344) 43 Net Ionic Equations 3. simplify by crossing out ions not directly involved (called spectator ions) Ag1+ + Cl1- AgCl This is called the net ionic equation Let’s talk about precipitates before we do some other examples 44 Predicting the Precipitate Insoluble salt is a precipitate [note Figure 11.11, p.342 (AgCl)] General solubility rules are found: a) Table 11.3, p. 344 in textbook b) Reference section - page R54 (Table B.9) 45 Let’s do some examples together of net ionic equations, starting with these reactants: 46 BaCl2 + AgNO3 → 47 NaCl + Ba(NO3)2 → 48 Pb(NO3)2(aq) + H2SO4(aq) 49