* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 2 Chemical Reactions
Inorganic chemistry wikipedia , lookup
Double layer forces wikipedia , lookup
Isotopic labeling wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Fine chemical wikipedia , lookup
Asymmetric induction wikipedia , lookup
Atomic theory wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical Corps wikipedia , lookup
Organic chemistry wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemical potential wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
History of chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical plant wikipedia , lookup
Drug discovery wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Marcus theory wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical industry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Click chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rate equation wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Chemical reaction wikipedia , lookup
Transition state theory wikipedia , lookup
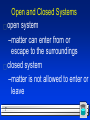
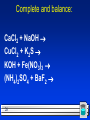
Chapter 2.2 Describing Chemical Reactions AISG Glenn Jacobsen 1 Section 2.2 Describing Chemical Reactions l OBJECTIVES: –Identify the information a chemical equation contains. 2 Section 2.2 Describing Chemical Reactions l OBJECTIVES: –State the principle of conservation of mass. 3 Section 2.2 Describing Chemical Reactions l OBJECTIVES: –Describe the steps for writing a balanced chemical equation. 4 Section 2.2 Describing Chemical Reactions l OBJECTIVES: –Name Five categories of chemical reactions. 5 All chemical reactions… have two parts: 1. Reactants = the substances you start with 2. Products = the substances you end up with Reactants Products 6 Open and Closed Systems open system –matter can enter from or escape to the surroundings closed system –matter is not allowed to enter or leave 7 In a chemical reaction Atoms can not be created or destroyed (Law of Conservation of Mass) A reaction can be described several ways: #1. In a sentence every item is a word Copper reacts with chlorine to form copper (II) chloride. 8 In a chemical reaction #2. In a word equation some symbols used Copper + chlorine copper (II) chloride #3. In a skeleton (not balance) equation symbols used Fe(s) + O2(g) Fe2O3(s) 9 #3 Symbols in equations? the arrow (→) separates the reactants from the products Read as: “reacts to form” or “yields” The plus sign = “and” (s) after the formula = solid: Fe(s) (g) after the formula = gas: CO2(g) (l) after the formula = liquid: H2O(l) 10 Symbols used in equations (aq) after the formula = dissolved in water, an aqueous solution: NaCl(aq) is a salt water solution 11 Now, read these equations: Fe(s) + O2(g) Fe2O3(s) Cu(s) + AgNO3(aq) Ag(s) + Cu(NO3)2(aq) Pt NO2(g) N2(g) + O2(g) 12 Balanced Chemical Equations Atoms can’t be created or destroyed in an ordinary reaction: A balanced equation has the same number of each element on both sides of the equation. 13 Rules for balancing: 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! 14 Rules for balancing: 4) If you have polyatomic ions that show up on both sides of the equations, replace them with a variable to make it easier. – Let X rep SO4 5) When dealing with O2 sometimes you have an odd number on one side. Put a ½ in front of the O2. Then balance and then multiply both sides by 2 6) Double-Check to make sure it is balanced. 15 Never change a subscript to balance an equation (You can only change coefficients) – If you change the subscript (formula) you are describing a different chemical. – H2O is a different compound than H2O2 16 Practice Balancing Examples _AgNO _Ag 2 3 + _Cu _Cu(NO3)2 + 2 _Mg + _N2 _Mg3N2 3 _P + _O 5 4 2 _P4O10 _Na + _H 2 2 2 2O _H2 + _NaOH _CH4 + _O 2 2 2O 2 _CO2 + _H 17 Types of Reactions We will learn: the 5 major types. 18 #1 - Synthesis Reactions Combine = put together 2 substances combine to make one Ca + O2 CaO SO3 + H2O H2SO4 We can predict the products Mg + N2 Mg3N2 19 #2 - Decomposition Reactions decompose = fall apart one reactant breaks apart into two or more compounds. electricity NaCl Na + Cl2 CaCO3 CaO + CO2 Note that energy (heat, sunlight, electricity, etc.) is usually required 20 #3 - Single Replacement Reactions One element replaces another Products will be a different element and a different compound. Na + KCl K + NaCl F2 + LiCl LiF + Cl2 21 (Cations switched) (Anions switched) #3 Single Replacement Reactions Practice: Fe + CuSO4 Pb + KCl Al + HCl 22 #4 - Double Replacement Reactions Two things replace each other. – Reactants must be two ionic compounds, in aqueous solution NaOH + FeCl3 – The positive ions change place. NaOH + FeCl3 Fe+3 OH- + Na+1 Cl-1 = NaOH + FeCl3 Fe(OH)3 + NaCl 23 #5 – Combustion Reactions Combustion means “add oxygen” Normally, a compound composed of only C, H, (and maybe O) is reacted with oxygen – usually called burning 24 #5 – Combustion Reactions If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just C) and H2O. 25 Complete and balance: CaCl2 + NaOH CuCl2 + K2S KOH + Fe(NO3)3 (NH4)2SO4 + BaF2 26 How to recognize which type? Look at the reactants: E + F = EF CD = C + D Synthesis Decomposition ED + C = EC +D Single replacement AB + CD = AD + CB Double replacement 27 SUMMARY: An equation... Describes a reaction Must be balanced in order to follow the Law of Conservation of Mass Can only be balanced by changing the coefficients. Has special symbols to indicate the physical state, if a catalyst or energy is required, etc. 28