* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Biochemical cascade wikipedia , lookup
Catalytic triad wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Butyric acid wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
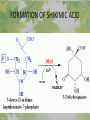
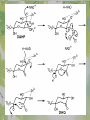
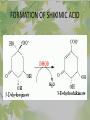
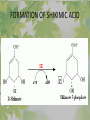
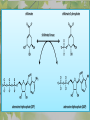
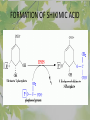
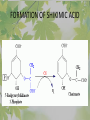
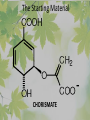
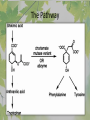
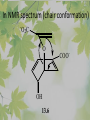
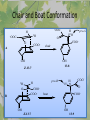
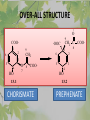
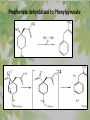
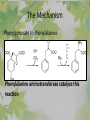
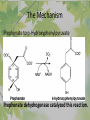
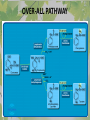
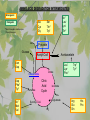
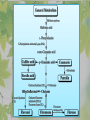
The Metabolic Pathway of Shikimic Acid Aromatic Amino Acids Phenolic Compounds Jan Michael O. Santos Philippine Normal University College of Science Department of Physical Sciences Outline of Discussion 1. Shikimic Acid Pathway a. b. c. d. e. Origin of Shikimic Acid Starting Material Enzymes Pathway- Mechanism Product 2. Aromatic Amino Acids a. b. c. d. e. What are the Aromatic Amino Acids Starting Material Enzymes Pathway- Mechanism Products 3. Phenolic Compounds a. b. c. d. e. What are Phenolic Compounds Starting Material Enzymes Pathway- Mechanism Products DEFINITION OF TERMS 1. Shikimic acid or shikimate: is an important biochemical metabolite in plants and microorganisms. 2. Aromatic compound: is a hydrocarbon with alternating double bonds and single bonds between carbon atoms 3. Amino acids: are molecules containing an amine group, a carboxylic acid group and a side chain (specific) 4. Phenolic Compounds: are a large and diverse group of molecules, which includes many families of aromatic secondary metabolite in plants. 5. Enzymes: are group of molecules that serve as a catalyst with a high degree of specificity for a certain substrate or class of substrates. It can only act on one substrate or on a family of structurally similar substrates. MAJOR ENZYMES 1. Synthase: Joints two molecules together w/o hydrolyzing a pyrophosphate bond. 2. Dehydratase: Removes water to create a double bond 3. Dehydrogenase: Removes hydrogen atom from its substrate 4. Kinase: Transfer a phosphate group from a high-energy phosphate compound such as ATP to its substrate. SHIKIMIC ACID Where this came from? Shikimi Illicium anisatum Shikimic acid is a precursor for: 1. Aromatic amino acids phenylalanine and tyrosine 2. Indole, and indole derivatives and a.a.a tryptophan 3. Alkaloids 4. Phenylpropanoids, flavonoids, tannins, and lignins. FORMATION OF SHIKIMIC ACID Starting materials 1st: Pyruvate to Phospoenolpyruvate FORMATION OF SHIKIMIC ACID 2nd : Erthryrose-4-phosphate FORMATION OF SHIKIMIC ACID Analyze this reaction, what are the other materials involve? FORMATION OF SHIKIMIC ACID 3-deoxy-D-arabinoheptulosonate 7phosphate (DAHP) synthase is the first enzyme in a series of metabolic reactions known as the shikimate pathway. Since it is the first enzyme in the shikimate pathway, it controls the amount of carbon entering the pathway. FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID phosphoenol pyruvate H H COOH O O HO P O C H PO CH2 HO P O HO H O H+ H CH2 B: CH2 O HO O C COOH H CH2 HO O OH H O HO H erythrose-4-phosphate H+ COOH HO COOH H+ O C COOH CH2 H NADPH H2C HO OH OH shikimic acid COOH O OH OH O H OH OH OH H O HO H FORMATION OF SHIKIMIC ACID 3-dehydroquinate synthase is the second enzyme of the shikimate pathway. It catalyzes the elimination of phosphate from DAHP to generate 3-dehydroquinate (DHQ). FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID 3-Dehydroquinate Dehydratase (DHQD) catalyzes the third step of the shikimate pathway, dehydration of 3-dehydroquinate to 3-dehydroshikimate. FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID Shikimate-5-Dehydrogenase (SDH) The fourth step of the shikimate pathway is the reduction of DHS to shikimate. the reaction is catalyzed by an NADP-dependent shikimate dehydrogenase (SHD) FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID Shikimate Kinase (SK)- In the fifth step of the shikimate pathway, shikimate kinase catalyzes the specific phosphorylation of the 3-hydroxyl group of D-shikimate to yield shikimate 3-phosphate using ATP as a cosubtrate FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID 5-Enolpyruvylshikimate 3-Phosphate Synthase (EPSPS) is the sixth enzyme of the shikimate pathway. It catalyzes the reversible formation of 5-enolpyruvyl-shikimate-3phosphate (EPSP) from shikimate 3phosphate and PEP. FORMATION OF SHIKIMIC ACID FORMATION OF SHIKIMIC ACID Chorismate Synthase (CS) The seventh and final step in the main trunk of the shikimate pathway is the trans-1,4 elimination of phosphate from EPSP to yield chorismate FORMATION OF SHIKIMIC ACID In this reaction, the second of the three double bonds of the benzene ring is introduced. The reaction is catalyzed by chorismate synthase and requires reduced flavin for activity even though the overall reaction is redox neutral. FORMATION OF SHIKIMIC ACID SHIKIMIC ACID TO AROMATIC ACID The seven-step shikimate pathway links the metabolism of carbohydrates to the biosynthesis of aromatic amino acids and many aromatic secondary metabolites, including tetrahydrofolate and ubiquinone. Aromatic Amino Acids Aromatic Amino Acids 1. What are aromatic amino acids? Aromatic Amino Acids are amino acids that include an aromatic ring. Example includes: Phenylalanine, Tryptophan, Histidine, Tyrosine (but only F, W, Y can be synthesized by Shikimate pathway) The Aromatic Family In plants and microorganism: Phe, Tyr, and Trp Precursors are: PEP ERYTHROSE-4-PHOSPHATE CHORISMATE The Starting Material CHORISMATE Enzymes Isomerase is an enzyme that catalyzes the structural rearrangement of isomers. Mutase: catalyzes the shifting of a functional group from one position to another within the same molecule. Transferase: catalyzes the transfer of a functional group (methyl or phosphate)from one molecule to another The Pathway The Mechanisms From Chorismate to Prephenate The Mechanisms What is Claisen Rearrangement? Claisen Rearrangement is a powerful carbon-carbon bondforming chemical reaction discovered by Rainer Ludwig Claisen. In NMR spectrum (chair conformation) -O 2C O OH 13.6 COO- Chair and Boat Conformation -OOC H 3H -OOC O COO- A COO- chair OH 13.8 Z-13.7 O B 3H O OH 3H pro-S H pro-R H COOCOO- 3H COO- H O COO- boat OH OH Z-13.7 13.9 OVER-ALL STRUCTURE 7 9 COO1 6 4 HO CH2 C -OOC 9 2 CH2 3 5 O O 8 COO- 7 13.1 CHORISMATE 1 6 5 8 COO- 2 3 4 HO 13.2 PREPHENATE Phenylalanine Biosynthesis Prephenate dehyrdatase to Phenylpyruvate The Mechanism Phenylpyruvate to Phenylalanine Phenylalanine aminotransferase catalyze this reaction The Mechanisms Prephenate to Phenylpyurvate to Phenylalanine SOURCES OF ESSENTIAL AMINO ACIDS Phenylalanine Tyrosine Biosynthesis The Mechanism Prephenate to p-Hydroxyphenylpyruvate Prephenate dehydrogenase catalyzed this reaction. The Mechanism p-Hydroxyphenylpyruvate to Tyrosine Tyrosine aminotransferase catalyzed this reaction SOURCES OF ESSENTIAL AMINO ACIDS Tyrosine OVER-ALL PATHWAY Tryptophan Biosynthesis The Mechanism This reaction is catalyzed by athranilate synthase The Mechanism This reaction is catalyzed by athranilate phosphoribosyl transferase The Mechanism This reaction is catalyzed by phosphoribosyl athranilate isomerase. The Mechanism This reaction is catalyzed by indole-3glucerol phosphate synthase. This reaction is catalyzed by tryptrophan synthase OVER-ALL PATHWAY SOURCES OF ESSENTIAL AMINO ACIDS Tryptophan SOURCES OF ESSENTIAL AMINO ACIDS Phenylalanine SOURCES OF ESSENTIAL AMINO ACIDS Tyrosine SOURCES OF ESSENTIAL AMINO ACIDS Tryptophan AMINO ACID DEGRADATION INTERMEDIATES Glucogenic Ala Cys Gly Ketogenic * Both Glucogenic and Ketogenic • Purely Ketogenic CO2 Glucose Ile* Leu• Lys• Thr* Ser Thr* Trp* Pyruvate Acetyl-CoA Acetoacetate Asn Asp Citrate Oxaloacetate Asp Phe* Tyr* Fumarate Leu• Lys• Phe* Citric Acid Cycle Trp* Tyr* Isocitrate CO2 Ile* Met Val Succinyl-CoA a-ketoglutarate CO2 Arg Glu Gln His Pro PHENOLICS These are the class of natural occuring compound with one or more phenolic compounds or benzene ring with –OH group. Quercetin, a typical flavonoid, is a natural phenol BIOSYNTHESIS OF PHENOLICS Most of the natural phenols are derived from secondary plant metabolism of the shikimic acid pathway, malic acid pathway or both. APPLICATIONS One very good example are HORSE GRAMS- a kind of beans SOURCES OF PHENOLIC ACIDS Horse Grams/ beans has the following kinds of phenolic acids: Caffeic Acid Protocatechuic acid p-coumaric acid Thank You for Listening!