* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture Resource ()
Discodermolide wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Elias James Corey wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Polythiophene wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
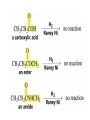
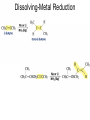
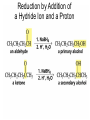
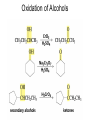
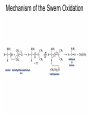
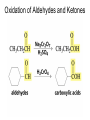
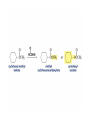
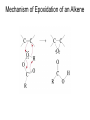
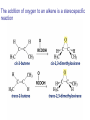
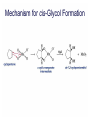
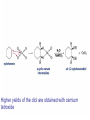
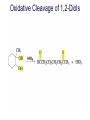
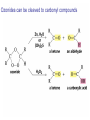
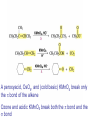
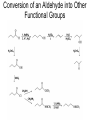
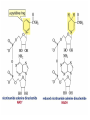
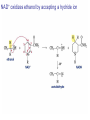
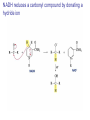
Organic Chemistry 4th Edition Paula Yurkanis Bruice Chapter 20 More About Oxidation–Reduction Reactions Irene Lee Case Western Reserve University Cleveland, OH ©2004, Prentice Hall • Oxidation is always coupled with reduction • Loss of electrons is oxidation • Gain of electrons is reduction • The oxidation state of a carbon atom equals the total number of its C–O, C–N, and C–X bonds Hydrogen, sodium borohydride, and hydrazine are the reducing agents Bromine and chromic acid are the oxidizing agents H2 as a Reducing Agent Reduction by Catalytic Hydrogenation Dissolving-Metal Reduction Reduction by Addition of a Hydride Ion and a Proton LiAlH4 is a stronger reducing agent than NaBH4 LiAlH4 is used to reduce compounds that are nonreactive toward NaBH4 DIBAL allows the addition of one equivalent of hydride to an ester Replacing some of hydrogens of LiAlH4 with OR groups decreases the reactivity of the metal hydride Formation of Amines by Reduction NaBH4 can be used to selectively reduce an aldehyde or a keto group in a compound Oxidation of Alcohols Oxidation of a Primary Alcohol Mechanism of Alcohol Oxidation by Chromic Acid The oxidation of a primary alcohol can be stopped at the aldehyde if pyridinium chlorochromate (PCC) is used as the oxidizing agent The Swern Oxidation Mechanism of the Swern Oxidation Oxidation of Aldehydes and Ketones The Tollens Reagent Oxidizes Only Aldehydes Both aldehydes and ketones can be oxidized by peroxyacid: The Baeyer–Villiger oxidation Mechanism of the Baeyer–Villiger Oxidation Therefore, the product of the Baeyer–Villiger oxidation of cyclohexyl methyl ketone will be cyclohexyl acetate, because a secondary alkyl group is more likely to migrate than a methyl group Oxidation of Alkenes with Peroxyacids Mechanism of Epoxidation of an Alkene The addition of oxygen to an alkene is a stereospecific reaction Hydroxylation of Alkenes Mechanism for cis-Glycol Formation Higher yields of the diol are obtained with osmium tetroxide Oxidative Cleavage of 1,2-Diols Oxidative Cleavage of Alkenes by Ozonolysis The alkene and ozone undergo a concerted cycloaddition Mechanism of ozonide formation The molozonide is unstable because it has two O–O bonds Ozonide is stable Ozonides can be cleaved to carbonyl compounds Examples of the Oxidative Cleavage of Alkenes by Ozonolysis A peroxyacid, OsO4, and (cold basic) KMnO4 break only the p bond of the alkene Ozone and acidic KMnO4 break both the p bond and the s bond Table 20.1 Summary of the Methods Used to Oxidize an Alkene Oxidative Cleavage of Alkynes O O KMnO 4 CH3C CCH2CH3 CH3C CCH2CH3 HO O CH3C CCH2CH3 O3 CH3COH O + CH3CH2COH H2O O CH3CH2CH2C CH O3 H2O CH3CH2CH2COH + CO2 Conversion of an Aldehyde into Other Functional Groups Conversion of a Ketone into an Ester or an Alcohol Biological Oxidation–Reduction Reactions NAD+ oxidizes ethanol by accepting a hydride ion NADH reduces a carbonyl compound by donating a hydride ion