* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic/Biological Chemistry
Survey
Document related concepts
Transcript
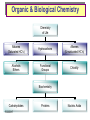
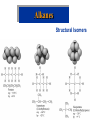
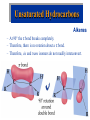
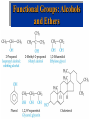
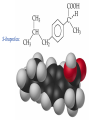
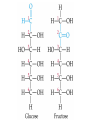
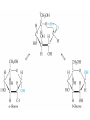
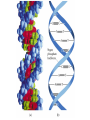
Organic & Biological Chemistry Chemistry of Life Alkanes (Saturated HC’s) Hydrocarbons Alkenes (Unsaturated HC’s) Alcohols Ethers Functional Groups Chirality Biochemistry Carbohydrates 5/23/2017 Proteins Nucleic Acids Some General Characteristics of Organic Molecules The Structures of Organic Molecules • Organic molecules exhibit three different types of hybridization at the carbon center: – sp3 hybridized carbons for tetrahedral geometries; – sp2 hybridized carbons for trigonal planar geometries; and – sp hybridized carbons for linear geometries. Drawing Lewis Structures 1. 2. 3. 4. 5. 6. Follow Step by Step Method (See Ng Web-site) Total all valence electrons. [Consider Charge] Write symbols for the atoms and guess skeleton structure [ define a central atom ]. Place a pair of electrons in each bond. Complete octets of surrounding atoms. [ H = 2 only ] Place leftover electrons in pairs on the central atom. If there are not enough electrons to give the central atom an octet, look for multiple bonds by transferring electrons until each atom has eight electrons around it. CyberChem video HyperChem Figure 9.3 HyperChem Summary of VSEPR Molecular Shapes e-pairs Notation Name of VSEPR shape Examples 2 AX2 Linear HgCl2 , ZnI2 , CS2 , CO2 3 AX3 Trigonal planar BF3 , GaI3 AX2E Non-linear (Bent) SO2 , SnCl2 AX4 Tetrahedral CCl4 , CH4 , BF4- AX3E (Trigonal) Pyramidal NH3 , OH3- AX2E2 Non-Linear (Bent) H2O , SeCl2 AX5 Trigonal bipyramidal PCl5 , PF5 AX4E Distorted tetrahedral (see-sawed) TeCl4 , SF4 AX3E2 T-Shaped ClF3 , BrF3 AX2E3 Linear I3- , ICl2- AX6 Octahedral SF6 , PF6- AX5E Square Pyramidal IF5 , BrF5 AX4E2 Square Planar ICl4- , BrF4- 4 5 6 HyperChem CyberChem video See Ng Web-site Methane Ethene Ethyne Figure 9.14: Covalent Bonding and Orbital Overlap Some General Characteristics of Organic Molecules sp3 Hybridzation in Carbon Bonding Introduction to Hydrocarbons • Hydrocarbons are compounds with only C and H. • Four classes: – – – – alkanes (all bonds and no bonds); alkenes (a mixture of and bonds, but no triple bonds); alkynes (must contain triple bonds); and aromatics (have planar, ring structures with alternating single and double bonds). • Saturated compounds have only bonds. • Unsaturated compounds have both and bonds. Alkanes HyperChem Alkanes Structural Isomers Alkanes Nomenclature Prefix What Substituent Base How many carbons Suffix What Family 1. Find longest continuous chain of carbon atoms, and use name of this chain (see Table 25.1) as the base name of the compound. 2. Number the carbon atoms in the longest chain, beginning with end of chain that is nearest to a substituent. 3. Name and give the location of each substituent group. 4. When two or more substituents are present, list them in alphabetical order. Alkanes Cycloalkanes Alkanes Reactions of Alkanes • The C-C and C-H bonds are very strong. Therefore, alkanes are very unreactive. • At room temperature alkanes do not react with acids, bases, or strong oxidizing agents. • Alkanes do combust in air (making them good fuels): 2C2H6(g) + 7O2(g) 4CO2(g) + 6H2O(l) H = -2855 kJ Unsaturated Hydrocarbons Alkenes • Geometrical isomers are possible since there is no rotation about a C=C bond. • Note the overlap between orbitals is above and below the plane of the bonds. – As the C-C bond begins to rotate (moving from cis to trans) the overlap decreases. Unsaturated Hydrocarbons Alkenes – At 90 the bond breaks completely. – Therefore, there is no rotation about a bond. – Therefore, cis and trans isomers do not readily interconvert. Unsaturated Hydrocarbons • • • • Alkynes Alkynes are hydrocarbons with one or more CC bond. Therefore, alkynes have one and two bonds between two C atoms. Ethyne (acetylene) is a reactive alkyne: HCCH. When acetylene is burned in the presence of oxygen (oxyacetylene torch) the temperature is about 3200 K. Alkynes are named in the same way as alkenes with the suffix -yne replacing the -ene for alkenes. Unsaturated Hydrocarbons Addition Reactions of Alkenes and Alkynes • The most dominant reaction for alkenes and alkynes involves the addition of something to the two atoms which form the double bond: H2C CH2 + Br2 H2C CH2 Br Br • Note that the C-C bond has been replaced by two C-Br bonds. Unsaturated Hydrocarbons Aromatic Hydrocarbons • Aromatic structures are formally related to benzene. • Benzene is not reactive because of the stability associated with the delocalized electrons. Functional Groups: Alcohols and Ethers Compounds with a Carbonyl Group Carboxylic Acids Chirality in Organic Chemistry • A molecule that exists as a pair of nonsuperimposable mirror images is called chiral. • Organic compounds that contain one carbon atom that is attached to four different atoms or groups are chiral. • The carbon atom attached to the four different moieties is called a stereogenic carbon. S-ibuprofen: Introduction to Biochemistry • Chemistry of living organisms is called biochemistry. • Biochemical molecules tend to be very large and difficult to synthesize. • Living organisms are highly ordered. Therefore, living organisms have very low entropy. • Most biologically important molecules are polymers, called biopolymers. • Biopolymers fall into three classes: proteins, polysaccharides (carbohydrates), and nucleic acids. Proteins Amino Acids • Proteins are large molecules present in all cells. • They are made up of amino acids. Video Summary Protein Structure • Pitch is the distance between coils. • The pitch and diameter ensure no bond angles are strained and the N-H and carbonyl functional groups are optimized for H-bonding. • Tertiary structure is the three dimensional structure of the protein. Carbohydrates • • • • • Carbohydrates have empirical formula Cx(H2O)y. Carbohydrate means hydrate of carbon. Most abundant carbohydrate is glucose, C6H12O6. Carbohydrates are polyhydroxy aldehydes and ketones. Glucose is a 6 carbon aldehyde sugar and fructose 6 carbon ketone sugar. • The alcohol side of glucose can react with the aldehyde side to form a six-membered ring. Carbohydrates Disaccharides • Lactose is formed from galactose and -glucose. • Sucrose is about six times sweeter than lactose, a little sweeter than glucose and about half as sweet as fructose. • Disaccharides can be converted into monosaccharides by treatment with acid in aqueous solution. Carbohydrates Polysaccharides • Bacteria in the stomach of animals contain cellulases, which are enzymes that enable animals to use cellulose for food. Nucleic Acids • Nucleic acids carry genetic information. • DNA (deoxyribonucleic acids) have molecular weights around 6 - 16 106 amu and are found inside the nucleus of the cell. • RNA (ribonucleic acids) have molecular weights around 20,000 to 40,000 amu and are found in the cytoplasm outside the nucleus of the cell. • Nucleic acids are made up of nucleotides. Organic & Biological Chemistry Chemistry of Life Alkanes (Saturated HC’s) Hydrocarbons Alkenes (Unsaturated HC’s) Alcohols Ethers Functional Groups Chirality Biochemistry Carbohydrates Proteins Nucleic Acids Saturated compounds have only bonds. Unsaturated compounds have both and bonds.