* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Batteries
Survey
Document related concepts
Transcript
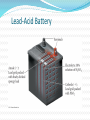
Commercial Voltaic Cells A voltaic cell can be a convenient, portable source of electricity. We know them as batteries. Batteries have been in use for over 100 years in various forms. The technology of batteries remained fairly stagnant until about 1990. Why??? Lead-Acid Battery This type of cell has been around for over 80 years. It uses lead as the anode and lead(IV) oxide as the cathode. Highly caustic H2SO4 is also involved in the overall reaction. The reaction produces a reliable 2.0 V. Lead-Acid Battery Lead-Acid Battery The half-reactions are: Pb(s) + HSO4-(aq) PbSO4(s) + H+(aq) + 2e (anode) PbO2(s) + 3H+(aq) + HSO4-(aq) + 2e PbSO4(s) + 2H2O(l) (cathode) Overall reaction is: PbO2(s) + Pb(s) + 2H+(aq) + 2HSO4-(aq) 2PbSO4(s) + 2H2O(l) During recharging, water is consumed. This used to require that water occasionally was added to the battery. The new batteries use Pb/Ca alloy as the anode which resists the consumption of water. This has led to the “maintenance-free” batteries. Lead-Acid Battery Advantages: produces steady voltage, very high current, many recharges, relatively low cost. Disadvantages: environmental concerns, massive, reverse reaction can produce H2. Zinc-Carbon Dry Cell Known also as the LeLanche cell (for its inventor), uses a zinc can as the anode and a graphite rod as the cathode. A paste containing NH4Cl and MnO2 separates the two electrodes. Zinc-Carbon Dry Cell The anode and cathode reactions are: Zn(s) Zn+2(aq) + 2e- (anode) 2 NH4+(aq) + 2 MnO2(s) + 2e- Mn2O3(s) + H2O(l) + 2 NH3(aq (cathode) Advantages: inexpensive, produces a reliable 1.5 V. Disadvantages: performs poorly under high demand, poor in cold weather, prone to leak when it gets old, environmental (disposal). The Zinc-Carbon Dry Cell Alkaline Dry Cell Similar, but uses KOH as the paste between the electrodes. The reactions are: Zn(s) + 2OH-(aq) Zn(OH)2(s) + 2e (anode) 2MnO2(s) + H2O(l) + 2e Mn2O3(s) + 2 OH-(aq) (cathode) Advantages: better under high demand, better in cold weather. Disadvantages: higher cost, environmental (disposal). Alkaline Dry Cell NiCad Cell Nickel-Cadmium (Nicad) batteries were some of the first widely used rechargeable batteries. The reactions are: Cd(s) + 2OH-(aq) Cd(OH)2(s) + 2e (anode) NiOOH(s) + H2O(l) + e Ni(OH)2(s) + OH-(aq) (cathode) Advantages: easy to recharge, many recharge cycles, good current supply. Disadvantages: longer recharge times, cost, weight, toxicity of Cd, and “memory loss.” NiMH Cell Newer version is the Nickel-Metal hydride (NiMH) battery that has longer life and eliminates the Cadmium which is replaced with a ZrNi2 metal alloy. This alloy absorbs Hydrogen anions that are oxidized. Most hybrid automobiles use these type of batteries. Advantages: Have a very long-life and can last for up to eight years. Disadvantage: Replacement costs in an auto can be upwards of $8,000. Lithium-Iodine Cell A “true” dry cell. The anode is lithium metal and the cathode is an I2 crystal. Current is carried by diffusion of Li+ ions. This battery is used in pacemakers as well as the BIOS in computers. Lithium-Iodine Cell The anode and cathode reactions are: Li(s) Li+(aq) + 1e (anode) I2(s) + 2e 2 I-(aq) (cathode) Advantages: environmentally friendly, produces a large voltage (3.0 V), long life, rechargeable, large power to mass ratio. Disadvantages: produces low current, cost. Lithium-Ion Cell A newer version of the previous type. Graphite serves as one electrode with LiCoO2 as the other electrode. During charging, the Li+ ions migrate to the anode (graphite) and the Cobalt is oxidized. During discharge, the Li+ migrate spontaneously to the cathode. These are the batteries of choice for most portable computers and PDA’s. Can be recharged many times for up to two years. Lithium-Ion Cell Lithium-Ion Cell Advantages: Store more energy per gram of weight, hold their charge of long periods, and each cell has a large voltage (3.6V). Disadvantages: Degrade even without use, last two to three years, cannot be completely discharged, and may catch fire if they fail. Cell Voltages / Currents Most devices require voltages of 3.0, 6.0, or even 12.0V as well as high currents. To produce these values, cells are placed in both series as well as in parallel. Fuel Cells Energy choice of the future. Not a true battery as it requires a constant supply of reactants. Used by NASA on space vehicles to generate electricity. May soon be mass produced for automobile propulsion. Smaller versions could power laptops and cell phones. Fuel Cells The overall reaction converts H2 and O2 into H2O. 2 H2(g) + 4 OH-(aq) 4 H2O(l) + 4e (anode) O2(g) + 2 H2O(l) + 4e 4 OH-(aq) (cathode) Overall Reaction is: 2 H2(g) + O2(g) 2 H2O(l) Fuel Cells Fuel Cells Fuel Cell Organization www.fuelcells.org Fuel Cell Producer / Researcher www.ballard.com Fuel Cells Advantages: best for the environment - produces water!, relatively low mass, much more efficient than the internal combustion engine, greatly simplify car design. Disadvantages: cost, storage / use of hydrogen, mass production, acceptance. Corrosion Electrochemical process of corrosion is essentially a mini voltaic cell. When a drop of water comes into contact with iron, the corrosion process begins. At the center of the drop, iron metal is oxidized: Fe Fe+2 + 2e. At the edges, oxygen is reduced: O2 + 4H+ + 4e 2H2O Corrosion Corrosion Corrosion of iron is more favored when: Moisture is present Concentrations of electrolytes (salt) is present Lower pH’s Prevention of corrosion can be achieved by: Paint – prevents oxygen and water from interacting with the iron Use of a sacrificial metal – any more active metal in contact with the iron will be oxidized in preference to the iron. This is sometimes called cathodic protection. Cathodic Protection Cathodic Protection