* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 17 and 18: Cellular Signaling Reference: Lieberman and
Endomembrane system wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Protein phosphorylation wikipedia , lookup
List of types of proteins wikipedia , lookup
Purinergic signalling wikipedia , lookup
NMDA receptor wikipedia , lookup
VLDL receptor wikipedia , lookup
Cannabinoid receptor type 1 wikipedia , lookup
Paracrine signalling wikipedia , lookup
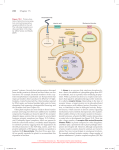
Lecture 17 and 18: Cellular Signaling Reference: Lieberman and Marks Chapter 11 1. Define a hormone, and describe differences between hormones that act intracellularly and extracellularly. A substance that is produced in one tissue or organ that is released into the blood and carried to another tissue or organ where it acts to produce a specific response. External chemical signals are received by receptors on the outside face of plasma membrane. o The receptors, which span the membrane, produce chemical signals on the inside of the cells. o These chemical signals propagate through the cell where they elicit specific cellular responses. o Some chemical signals enter the cell directly, and bind to an intracellular receptor, which has direct effects on cell processes (i.e., DNA). o Note: Kinases usually mediate receptors 2. Describe the fundamental differences between extracellular and intracellular signaling. Extracellular: typically bind peptide/protein hormones (e.g., insulin, growth hormone, parathyroid hormone) and tyrosine-derived catecholamines (dopamine, norepinephrine, epinephrine). o Almost always act to stimulate a signal cascade, whereby proteins are activated, second messengers are produced, and many cellular proteins are affected. Intracellular: typically bind steroid hormones (glucocorticoids like cortisol, mineralocorticoids (like aldosterone), androgens and estrogens, and Vitamin D), retinoic acid derivatives and thyroid hormones. o Almost always act at the DNA level, altering transcription of genes (i.e., turn on or turn off expression of a given protein, etc.) 3. List the two types of receptors, list a few examples of hormones they bind, and describe their general function (see the above question) 4. List and define the six basic steps of signaling. 1. Recognition of the hormone signal There must be a specific receptor for recognition 2. Transduction of the signal across the membrane This is done by a series of proteins and the receptor undergoes a conformational change 3. Transmission to intracellular components The receptor activates adaptor proteins 4. Modulation of the effector This is modulated by an effector molecule or secondary messenger 5. Response of the cell to the signal Cell status changes 6. Termination of the signal Degrades second messangers, turns off target proteins This signal needs to eventually disappear. This usually happens fast, especially in metabolic pathways, but a little slower in carbohydrates 5. Define the way cells communicate in terms of endocrine, paracrine and autocrine signaling. Endocrine: source of hormone and target of hormone far apart. Paracrine: source of hormone and target of hormone adjacent. Autocrine: cell produces and receives its own signals. 6. List the 5 basic types of receptor and list the three types of plasma membrane receptor. Types of receptors o Ligand Example: Acetylcholine with nicotinic acetylcholine receptor o G-protein Example: The Glucagon Receptor (heptihelical) o Intracellular (steroid) o Transmembrane proteins that release transcription factors o Catalytic Plasma membrane receptors o Ion channel receptors o Receptors that are kinases or bind kinases o Heptihelical (7-membrane spanning helices) receptors 7. Explain the function of a ligand-gated ion channel using the acetylcholine receptor as an example. NOTE: the level of detail on slide 12 is sufficient depth. An electrical signal on an axon causes the release of Acetylcholine (Ach) Acetylcholine (Ach) binding to the Ach receptor (AchR) stimulates a conformational change in the receptor. o Nicotinic acetylcholine receptor Has 5 subunits, where each of them are composed of 4 membrane spanning helical region Has two identical α subunits where Ach binds causes conformational change A lack of this receptor can cause Myasthenia Gravis, and autoimmune disease where Ab cross-link receptors so that less of them can activate and be functional This opens a channel, and this allows a rush of Na+ ions into the cell causing depolarization of the cell, and allows K+ to then flow out = Action Potential o Leads to the contraction of a muscle fiber The Ach signal is terminated by acetylcholinesterase, which degrades Ach. 8. Diagram and explain the mechanism of signaling for G-protein coupled receptors using the glucagon receptor as an example. Glucagon is a hormone that is secreted by pancreatic cells of Langerhans in the liver in response to fasting. Because you need energy because of this fasting state, this will oppose the action of insulin (because that takes energy) Glucagon tells cells to turn on pathways that convert energy stores to usable foods and turn off those pathways that need to build up energy stores. This is how blood glucose will still be maintained through the activation of glycogenolysis and gluconeogenesis. This breakdown only occurs in the liver Mechanism: o Glucagon binds to the G-protein coupled receptor in hepatic cells G-protein catalytic subunit (α) is inactive when GDP is bound Hormone binding to receptor induces conformational change in receptor that is communicated physically to α subunit Stimulates exchange of GDP for GTP, which activates the α subunit and causes it to release the βγ complex, which activates them. o Once bound, this causes GDP GTP which then acts on adenylate cyclase Activated α subunit diffuses to and binds to its target: adenylate cyclase This activates adenylate cyclase, which begins to produce cAMP (next step) Change in adenylate cyclase stimulates hydrolysis of GTP to GDP on α subunit Presence of GDP causes α subunit to leave the receptor and re-bind its other subunits βγ , reforming the complex α subunit is turned off until another receptor event. o Adenylate cyclase causes ATP cAMP o cAMP activates protein kinase A protein kinase A has four subunits, two regulatory (R) subunits, and two catalytic (C) subunits cAMP binds to the regulatory (R ) subunits on protein kinase A, releasing 2 catalytic subunit (C) the activate C units phosphorylate target proteins using ATP (see next step) o Protein Kinase A phosphorolyates specific target enzymes for fuel mobilization pathways = activates those that will fuel utilization and inhibits those for fuel storage Examples of increasing cycles: glycogenolysis, glyconeogenesis, lipolysis, ketogenesis, uptake of aa Example of decreasing cycles: glycogenesis o The signal will be quenched over time by the breakdown of cAMP cAMP phosphdiesterase breaks the cyclic bond to produce AMP, which does not have signaling properties. Decreased cAMP levels cause PKA R subunit to rebind to C subunit, inactivating it No additional phosphorylation takes place Phosphatases remove the phosphates from target proteins Thus, the balance between Adenylate Cyclase and cAMP phosphodiesterase controls the strength and duration of the signal. 9. Explain the concept of signal cascade amplification. One hormone activates one receptor that results in the generation of one second messenger molecule that diffuses within the cell to activate many target proteins. In the example of glucagon to the G-protein coupled receptor, one hormone binds to the one receptor activating the one adenylate cyclase, which produces lots of cAMP, which acts as a secondary messenger activating lots of pKA, which phosphorylates lots of target protein enzymes for fuel utilization 10. Explain the effect of cAMP on gene expression, including CRE, CRB and CREB. cAMP can also affect gene regulation cAMP response element binding protein (CREB) binds cAMP binding protein (CBP) Forms complexes at cAMP response element (CRE) and initiate gene transcription 11. List three other protein targets of G-protein signaling pathways. Phosphodiesterase (PDE) o Deals with adenylate cyclase o Insulin lowers cAMP levels by causing activation of physodiesterase Phospholipase C (PLC) Phospholipase D (PLD) 12. Define the action of phospholipase C and phospholipase D. Action of PLC through Gaq: o Gq stimulates(phospholipase C) PLC-β o PLC-β hydrolyzes phosphotidyl inositol bis-phosphate (PIP2)in to two second messangers: IP3 (1,4,5 - inositol triphosphate) DAG(diacylglycerol) o DAG-activated PKC stimulates IP3 release o IP3 releases Ca 2+ from intracellular stores Gas can activate PLC or PLD o PLC can convert phosphotydylcholine (PC) to phsophocholine and DAG o PLD can convert PC to choline and phosphatidic acid (PA) 13. Explain the structure and function of receptor kinases, and give three examples. Receptors composed two subunits, initially separated. o Can be heterodimers or homodimers. Hormone binding stimulates association of the two subunits, which activates the recptor, resulting in phosphorylation of the receptor o Can be autophosphorylation (e.g., tyrosine kinase receptors) o Can by phosphorylated by a kinase that binds (JAK-STAT receptors) The phosphorylation sites become docking sites for adaptor proteins (cofactor). Adaptor protein binding to the phosphorylated receptor results in activation of the adaptor protein. Activated adaptor proteins leave the receptor and bind cellular targets to change cellular state. Examples: o Tyrosine Kinase Receptor Growth factor binds to the receptor Dimerization occurs Tyr domains are autophosphorolyated Grb2 & SOS bind SOS binds Ras – GDP Ras exchanged GDP for GTP Ras – GTP binds Raf = active Raf – 1 phorphorolyates MEK MEK phosphorolyates MAPK MAPK phosphorolyates others MAPK translocates to the nucleus Phosphorolyation of transcription factors Alters gene expression o Jak – Stat Receptor Tyrosine associated receptor frequently used by cytokines to regulate proliferation of certain cells involved in the immune response. The receptor has no intrinsic kinase activity but it binds to the tyrosine kinase JAK (janus kinase) Their signal transducer proteins are called STATs (signal transducer and activator of transcription); these represent gene-specific transcription factors. Mechanism: Each receptor has an extracellular domain, a membrane spanning region, and the intracellular domain Cytokine binding results in dimer formation The activated JAKs phosphorylate each other and intracellular tyrosine residues on the receptor forming phosphotyrosinebinding sites for the SH2 domain of STAT Activated (phosphorylated STATs) dissociate from the receptor. STATs dimerize with other STATs and translocate into the nucleus. Binding to a DNA response element regulates gene expression o Faulty JAK-STAT Mutations affecting JAK2 cause a “gain of function” that results in a myeloproliferative disorder known as polycythemia vera. This disease is characterized by erythrocytosis (excessive erythrocytes or red blood cells) in the serum. abnormal proliferation of all hematopoietic bone marrow elements and an absolute increase in red cell mass and total blood volume o Serione/Threonine Kinase Receptor 14. Describe the structure of the insulin receptor and how it differs from typical receptor kinases. Member of the tyrosine kinase family receptor Unlike other growth factor receptors, it exists as a preformed dimer, each half has an α and β subunit. Insulin binding stimulates the receptor, which autophosphorylates o The phosphorylated receptor binds the insulin receptor substrate, and IRS gets phosphorylated by the receptor (β subunits autophosphorolyate) Phosphorylated IRS then activates target proteins: Grb2, phospholipase C-γ and phosphatidyl inositol 3-kinase 15. Explain the basic mechanism of action for the insulin receptor at the level of detail provided on slides 29 and 31. (see above) 16. Explain the difference between phosphatidyl inositol, PI-4,5-bisP, IP3, DAG, and PI3,4,5-trisP, and give an example of a typical function for each of these molecules. Phosphyatidylinositol Phosphates serve two functions in signal transduction: o PI-4,5-bisP can be cleaved by PLC to form IP3 and DAG (signaling molecules) o PI-4,5-bisP can be phosphorylated by PI 3-kinase to form PI-3,4,5-trisP (Docking site for signal transduction proteins.) Note prior to insulin binding, PI-3 kinase is quiescent and PI-4,5bisP is not a substrate for PDK-1 or PKB Insulin binding to the receptor activates IRS, which activates PI-3 kinase. The active PI-3-kinase phosphorylates PI-4,5-bisP, forming PI3,4,5-trisP, which binds and activates PDK-1 and PKB. Active PKB enters the cell cytoplasm, propagating the insulin signal 17. Describe in general an intracellular receptor and its mechanism of signaling. Intracellular receptors are receptors located inside the cell rather than on its cell membrane. o Examples are the class of nuclear receptors located in the cell nucleus and the IP3 receptor located on the endoplasmic reticulum. The ligands that bind to them are usually intracellular second messengers like inositol trisphosphate (IP3) and extracellular hormones like steroid hormones. They almost always act at the DNA level, altering transcription of genes 18. List the five hormones that typically bind to intracellular receptors. The steroid or thyroid hormones: o Cortisol o Aldosterone o Thyroid hormone (T3) o Vitamin D3 o Rectinoids 19. Define the terms transactivation and response element. Transactivation- ligand induced conformational change of the receptor that results in its binding to a specific segment of DNA to initiate (or in some instances to block) transcription. The DNA segments are called response elements. o Ex. The steroid response element (SRE) or the cAMP response element (CRE). 20. List several examples of second messengers in extracellular signaling cAMP IP3 21. Explain the mechanism for intracellular signaling, including the steps for hormonereceptor-gene regulation. After hormone enters cell (via diffusion or carrier mediated) it bind to receptor causing dimerization o Hormone may bind to the receptor before or after dimerization Receptor/hormone binds to specific place on DNA called hormone response element and activate cis linked genes Hormone receptor unbound in cytoplasm coupled to hsp90 o Once it goes into the nucleus through the nuclear localization signal hps90 is released 22. Describe how pertussis toxin and cholera toxin affect cellular signaling. Cholera Toxin o Cholera toxin a secretory product of bacterium vibrio cholerae o Toxin catalyzes the adenosine diphosphate (ADP-) ribosylation of Gαs (an allosteric covalent modification). o ADP-ribosylation inhibits the GTPase activity of Gαs, so Gαs remains in its activated GTP-bond form, and continuously activates adenylate cyclase in epithelial cells. o The constitutively activated Gαs elevates levels of cAMP which causes an increase in Cl- conductance and water flow and thereby contributing to the large fluid loss characteristic of the disease. Pertussis Toxin o Pertussis Toxin is produced by bordetella pertussis, the causative agent of whooping cough. o Pertussis toxin ADP ribosylates Gαi o ADP ribosylation of Gαi cannot exchange its bound GDP for GTP. o The ADP-ribosylated Gαi remains in its GDP bound, inactive state and therefore cannot inactivate adenylate cyclase. o cAMP levels stay higher than normal under resting conditions.