* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 15
Neutron magnetic moment wikipedia , lookup
Mathematical descriptions of the electromagnetic field wikipedia , lookup
Magnetosphere of Jupiter wikipedia , lookup
Geomagnetic storm wikipedia , lookup
Giant magnetoresistance wikipedia , lookup
Maxwell's equations wikipedia , lookup
Magnetosphere of Saturn wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Earth's magnetic field wikipedia , lookup
Magnetoreception wikipedia , lookup
Van Allen radiation belt wikipedia , lookup
Magnetic monopole wikipedia , lookup
Electromotive force wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Electromagnet wikipedia , lookup
Multiferroics wikipedia , lookup
Magnetotellurics wikipedia , lookup
Electromagnetism wikipedia , lookup
Lorentz force wikipedia , lookup
Electromagnetic field wikipedia , lookup
Superconducting magnet wikipedia , lookup
History of electrochemistry wikipedia , lookup
Electricity wikipedia , lookup
Force between magnets wikipedia , lookup
Electric charge wikipedia , lookup
Magnetochemistry wikipedia , lookup
Ferromagnetism wikipedia , lookup
Static electricity wikipedia , lookup
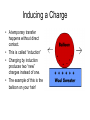
Chapter 15 Electricity and Magnetism History of Electrostatics • Electrostatics is the study of the nature, behavior, and uses of static electricity. • Benjamin Franklin is famous for his study of electricity! The Study of Static Electricity The Electric Charge: We know that all matter consists of tiny particles called atoms which contain protons, neutrons, and electrons. Protons and electrons have a certain amount of “electric charge.” This electric charge enables them to attract and repel each other. The Study of Static Electricity The Electric Charge cont… Protons are positive (+) Electrons are negative (-) Electricity is the result of the transfer of electrons. The Study of Static Electricity Ordinarily – the charges are in equal balance and the atom is said to be “neutral.” If an atom loses an electron or gains an electron, it is “charged”.. Too many electrons? It is negatively charged. Too many protons? It is positively charged. The Study of Static Electricity Opposite charges neutralize each other!!! One of the ways to cause an atom to gain or lose electrons is with friction. When an objects “rub” against each other, the electrons can “rub off” one object onto another. The Study of Static Electricity • Whenever charges are built up in two objects by friction, one object is charged negatively and the other is charged positively. Electric Fields • We cannot see electric fields – we CAN map them with imaginary lines. • These lines are called “lines of Force” Measuring Charge • The more electrons an object gains or loses, the stronger its charge will be. • The unit of charge is the “coulomb” • One coulomb equals 6.24 billion billion electrons. (6.24 X 1018) Electrostatic Laws • Law of electric charges: opposite charges attract each other, like charges repel each other • Law of electric force: strong charges attract strongly, weak charges attract weakly Electrostatic Laws • Coulomb’s Law of Electric Force - strength of the attraction or repulsion is directly related to the strength of the charges and inversely related to the distance between them. Coulomb’s Law of Electric Force The force between two charges is 100 Newtons 1. What happens if the distance d is doubled? 2. Double both charges? 3. Double d and charges? Sharing a Charge Contact – neutral object touches a charged object – charged object transfers the charges. This can happen with positive and negatively charged objects. Charging by contact is permanent! The transfer of charges is conserved – that is the amount of charge is the same before and after the transfer. Transferring Charges • An object’s charge can be taken away completely by bringing it into contact with the ground! • The earth is so “huge” it can soak up an object’s electrical charge. • This is called grounding! Inducing a Charge • A temporary transfer happens without direct contact. • This is called “induction” • Charging by induction produces two “new” charges instead of one. • The example of this is the balloon on your hair! Detecting Charges • Small charges can be detected by means of an electroscope. • When uncharged, the foil leaves stay down. • When charged, the foil leaves rise up. Electrostatic Generators • The Van de Graaff Generator • The charges move outward! Protection from Lightning • Benjamin Franklin knew that if the charge from a lightning strike could be directed into the ground, then the strike would be harmless. • So, He invented the Lightning Rod. Protection from Lightning • Franklin’s famous experiment is VERY DANGEROUS! • Numerous people over the years have tried to duplicate it and have died from being struck by lighting! • Do not attempt! Video on Static Electricity http://streaming.discoveryeducation.com/sea rch/assetDetail.cfm?guidAssetID=bbb0d71 7-46f7-4fed-94350db35d27cae6&tabDisplay=myContent Magnets and Magnetism • The first magnet ever used was actually a lodestone which is a natural magnet. • It is a form of the mineral magnetite! Magnets and Magnetism The Vikings were the first to use lodestones for navigation. The Nature of Magnets • Magnets come in various sizes and strengths. • Magnets are used in televisions and speakers and computers. Magnetic Fields • The property of attracting objects by the magnetic force is known as magnetism. • The region around a magnet that other objects are attracted to are the magnetic field. • Compasses are affected by these fields. • The iron filings represent lines of force. Law of Magnetic Poles • All magnets possess poles. • One pole always seeks North. • One pole always seeks South. • The Law of Magnetic Poles states: Like poles repel and unlike poles attract. Law of Magnetic Poles http://www.windows.ucar.edu/tour/link=/physi cal_science/magnetism/bar_magnet_inter active.html Permeability • Some substances can absorb or channel the lines of magnetic force better than others. • The ability to do this is permeability. • Highly permeable substances include iron, cobalt, and nickel. The Earth as a Magnet • The earth’s magnetic field reaches out into space thousands of miles! • We don’t know why we are a magnet…scientists think it may be that we have electricity circulating in the core. The Earth as a Magnet • The magnetic poles do not coincide with the geographic north and south poles. • The poles wander about 5 miles every year! The Magnetosphere • The earth’s magnetic field out in space is called the magnetosphere. • The magnetosphere is distorted by the solar wind. • The magnetosphere protects the earth from the harmful effects of the sun. Van Allen Radiation • Some of the sun’s radiation is trapped in the magnetosphere. • These trapped particles are called Van Allen belts. Aurora Borealis • Some particles of solar wind near the poles sometimes funnel down toward the earth. • They crash into particles of air and produce brilliant shimmering lights known as the Aurora Borealis. • The frequency of these depends on the solar winds. Aurora Borealis Understanding Magnets • Magnets are caused by the spin of electrons around an atom. • As a rule, the more unpaired electrons an atom has the more likely it is magnetic. Understanding Magnets • Domains – A group of aligned atoms having a single magnetic field is a domain. – A small bar magnet will have thousands of domains. – A magnetic substance forms a magnet only if its domains are mostly aligned. Types of Magnetic Materials • Diamagnetic – no unpaired electrons • Paramagnetic – one unpaired electron • Ferromagnetic – several unpaired electrons. Methods of Magnetizing • Magnetizing by contact – may be temporary or permanent. • Magnetizing by induction – no contact needed. • Magnetizing by electricity – electricity flows through a wire and produces a magnetic field. The Electromagnet • An iron core with a wire wrapped around it can become a magnet if the wire has a current flowing through it. The End!!!!