* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Our focus is on vaccines
Hygiene hypothesis wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Vaccination policy wikipedia , lookup
Immune system wikipedia , lookup
Herd immunity wikipedia , lookup
Thiomersal controversy wikipedia , lookup
Innate immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Whooping cough wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Hepatitis B wikipedia , lookup
DNA vaccination wikipedia , lookup
HIV vaccine wikipedia , lookup
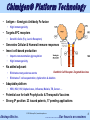
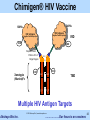
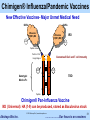
Akshaya Bio, Inc. Chimigen® Technology Re-educating Immune System to Fight Diseases © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 1 Outline The Company Management Advisors & Consultants R&D Team Chimigen® Platform Technology Product Pipeline & Status Issued Patents Akshaya’s Competitive Advantages Highlights Contact Information © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 2 Akshaya Bio Inc. – The Company • Based in Edmonton, Alberta, Canada – Currently a Team of Seven, Six Researchers (5 Ph.Ds, 1 B.Sc, 1 MBA) – Privately held • Founded in 2010 – Dendritic cell receptor-targeted vaccines – Proprietary technology • 23 Issued Patents • 17 patents pending worldwide – Acquired Chimigen® Technology from Paladin Labs Inc. (TSX) and ViRexx Medical Corp. (TSX, AMEX) • External Partners in Research and Development – NRC-IRAP, CHTD, AITF, Gates Foundation • Unique technology platform • Improved antigen presentation – – “Mimics Body’s Own Natural Immune Response Process” No adjuvant, avoids adverse reactions • Rich product pipeline – HBV, HCV, HIV, Cancer, Malaria, Biodefense, Influenza, TB © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 3 Experienced Management • Satish Chandran, Ph.D CEO: biotech/vaccine veteran with 25+ years leadership experience; ex Pfizer, Wyeth-Lederle vaccines • Rajan George, M.Sc, Ph.D President and Chief Scientific Officer: 24+ years R&D experience in virology, immunology, molecular biology; ex Paladin Biosciences E.VP and CTO, ViRexx Medical Corp. Senior VP • Bruce Hirsche, QC General & IP Counsel • Rohit R. George, BA, MBA Business Operations © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 4 Advisors & Consultants Robert Gish, MD Hepatologist, Clinical Director-Hepatitis B Foundation Frank Chisari, MD Scripps Institute Natl. Acad. Member Leading Expert in HBV and HCV Timothy Block, Ph.D. Director Inst. Hepatitis & Virus Res., Professor, Drexel U President Hepatitis B Foundation Richard Ascione, MD, Ph.D Professor, Georgetown University, Washington DC Fairlook Investment Advisors LLC, New York Christopher Walker, Ph.D Director, Center for Vaccines and Immunity, Nationwide Children's Hospital, Columbus, Ohio Expert in HCV immunotherapy © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 5 Research Team • Allan Ma, Ph.D • Senior Scientist, Virologist • Dakun Wang, Ph.D • Senior Scientist, Immunologist • Gina Thede, Ph.D • Biochemist/Molecular Biologist • Hue Anh Luu, B.Sc • Technologist © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 6 Chimigen® Platform Technology A Novel Vaccine Technology for Infectious Diseases and Cancer Dendritic Cell Receptor-Targeted Vaccines “Mimics Body’s Own Natural Immune Response Process” © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 7 Chimigen® Platform Technology • Antigen – Xenotypic Antibody Fc-fusion • • High immunogenicity Targets APC receptors • Dendritic Cells (Fcγ, Lectin Receptors) • Generates Cellular & Humoral immune responses • Insect cell-based production • • • Imparts non-mammalian glycosylation • High immunogenicity No added adjuvant Dendritic Cell Receptor-Targeted Vaccines • Eliminates many adverse events • Eliminates T cell sequestration, dysfunction & deletion Adaptable platform • HBV, HCV, HIV, Alphaviruses, Influenza, Malaria, TB, Cancer … • Potential use for both Prophylactic & Therapeutic Vaccines • Strong IP position: 23 issued patents, 17 pending applications © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 8 Akshaya Bio Inc. Product Pipeline….. © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 9 Product Candidates & Status Product Discovery Pre-Clinical Phase I Phase II Chimigen® HBV Vaccine Chronic Hepatitis B Ready for licensing Chimigen® HCV Vaccine Hepatitis C Ready for licensing Chimigen® HIV Vaccine HIV Prevention/ Therapy Cancer Vaccines Various Targets Chimigen® Malaria Vaccine Malaria Prevention/ Therapy Alphaviruses (WEEV) Biodefense Influenza H5 & H1 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. Phase III …..…………..Our focus is on vaccines 10 Akshaya Bio Inc. HBV Therapeutic Vaccine: Treatment for Chronic HBV Infection © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 11 Hepatitis B Virus (HBV) Infection Shocking epidemiology:380 million chronic carriers HBsAg Prevalence >=8% - High 2-7% - Intermediate <2% - Low As many as 140 million people will die from HCC or cirrhosis due to HBV without intervention From Murray et. al., Medical Microbiology 5th edition, 2005, Chapter 66, published by Mosby Philadelphia © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 12 HBV Therapeutic Vaccine Significant Unmet Need • Considerations • Over 380 million chronically infected (~2 million in US) • One million deaths annually – Cirrhosis, Liver failure & HCC • Current Antiviral Therapies • Polymerase Inhibitors • Effective in 30-40% of chronically infected patients • Mutant (resistant) strains appear frequently 6xHis • Compliance issues with interferon treatment • • CHO IRD CHO Peptide Linker Contains T & B cell-relevant HBV antigens Able to elicit humoral and cellular response Potential prophylactic and therapeutic uses Ready to enter clinical studies In discussions to out license Accepted by NIH for Product Development Plan © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. HBV S1/S2/Core HBV S1/S2/Core Market: >$2B Akshaya’s HBV Therapeutic Vaccine • • • • • • 6xHis Portion of CH1 S-S Hinge Region S-S SS CHO CH2 S S CCH2 H2 Xenotypic Murine Fc CHO TBD SS SS CH3 CH3 Peptide …..…………..Our focus is on vaccines 13 Chimigen® HBV Therapeutic Vaccine Efficacy, ex vivo PBMCs derived from individuals un-infected and with chronic HBV infection © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 14 Chimigen® HBV Therapeutic Vaccine Summary • Binds to immature DCs – CD32 & CD206 • Appropriate antigen processing and presentation – Efficacy in Normal and HBV carriers, ex vivo in DC/T cell antigen presentation assays • Induces activation and proliferation of CD8+ and CD4+ T cells • Production of IFN-γ and TNF-α in CD8+ and CD4+ T cells • Production of granzyme B in CD8+ & CD4+T cells • Production of HBV antigen-specific CD8+ T cells (Pentamer Positive) • HBV peptide-specific T cell responses (IFN- and TNF- ) • Targeted cell killing – HBV antigen-loaded Un-infected & HBV Chronic carrier cells • Induces T(resp) cells, removes T(reg) cells © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 15 Chimigen® HBV Therapeutic Vaccine Immunology, in vivo Cellular and Humoral Responses in Sheep © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 16 Chimigen® HBV Therapeutic Vaccine Results Summary, in vivo • Chimigen® HBV Therapeutic Vaccine is immunogenic in sheep • Chimigen® HBV Vaccine induced a dose-dependent antibody response • Specificity for S1/S2 protein • Significant response following the secondary immunization • Kinetics of the antibody response similar to commercial HBV sAg vaccine (Engerix-B, GSK), but with no added adjuvant • Chimigen® HBV Vaccine induced a dose-dependent cell-mediated immune response • Specificity for S1/S2 protein • Significant response following the secondary immunization • Effective at low doses • Systemic immune responses in blood was higher than the local immune response in the lymph node draining the site of vaccination • Induces immune activation in HBV-transgenic mice (NIH Study) • HBV Vaccine is induces systemic cell-mediated immune response © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 17 Chimigen® HBV Core Therapeutic Vaccine Immunology, in vivo HBV Transgenic Mice © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 18 Chimigen® HBV Core Therapeutic Vaccine Chimigen® HBV Core Therapeutic Vaccine in HBV Transgenic Mice Induced Immune Responses in the Liver Following Intradermal Injections Study Carried out by NIH Expt. No. NHA-77 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 19 Chimigen® HBV Vaccine Therapeutic Value • Immune Responses are indicative of breaking tolerance in HBV chronic carriers • These novel responses are indicative of administering a therapeutic effect – Production of new epitope-specific CTLs – The Killing of HBV-infected target cells – Production of T responder cells – Killing of T regulatory cells by T responder cells – Cytokine induction in HBV transgenic mice © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 20 Akshaya Bio Inc. Chimigen® HCV Vaccine: Prophylactic/Therapeutic Vaccine © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 21 Hepatitis C Virus (HCV) Infection • Considerations • HCV is a global health care problem (WHO) • 3-4 million new infections each year • 170 million chronically infected • 2.7 million in US • Cirrhosis and loss of liver function, HCC • 6xHis 6xHis HCV E1-E2-NS5A Current Therapies HCV E1-E2-NS5A CHO CHO • Antivirals, Interferon α, Polymerase and Protease inhibitors IRD Peptide Linker Portion of CH1 • Effective in select viral strain infections and groups S-S Hinge Region S-S SS • High costs CHO CH2 S S CCH2 H2 CHO Xenotypic Fc • Severe side effects with interferon treatment – compliance issues TBD SS • Continued emergence of drug resistant mutants SS CH3 CH3 Peptide Contains two B cell and one T-cell relevant HCV antigens • Akshaya’s HCV vaccine is designed for prophylactic and therapeutic applications • Design and process development completed • Preclinical studies completed • Ready for clinical development © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 22 Chimigen® HCV E1-E2-NS5A Vaccine 6xHis 6xHis HCV E1-E2-NS5A HCV E1-E2-NS5A IRD CHO CHO Peptide Linker Portion of CH1 S-S Hinge Region S-S SS CHO CH2 SS CHO CCH2 H2 Xenotypic Fc TBD SS SS CH3 CH3 Peptide HCV Prophylactic/Therapeutic Vaccine © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 23 Chimigen® HCV E1-E2-NS5A Vaccine Summary • Purified and characterized • High Yield ~20mg/Litre • Binds to immature Dendritic Cells • Vaccine-loaded DCs HCV naïve individuals induce: •Proliferation of CD8+ and CD4+ T cells •Increase in the proportion of CD8+ and CD4+ T cells producing the Th1 cytokines IFN-γ and TNF-α •Proliferation of CD19+CD138+ B cells •Proliferation of CD19+CD38+ B cells • Induces both T cell and B cell immune responses • Excellent potential to develop Prophylactic/Therapeutic Vaccine © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 24 Chimigen® HIV Vaccine 6xHis 6xHis HIV antigens HIV antigens Gag/Env/Tat/Rev/Vpr/Vpu Gag/Env/Tat/Rev/Vpr/Vpu CHO IRD CHO Peptide Linker Portion of CH1 Hinge Region S-S S-S S S Xenotypic (Murine)Fc CHO CHO CH2 S S CCH2 H2 TBD S S CH3 S S CH3 Multiple HIV Antigen Targets © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 25 Chimigen® HIV Vaccine Immune Responses, ex vivo • Binding to Dendritic Cells • Dendritic Cell / T Cell Antigen Presentation Assay (APA) ex vivo Human Peripheral Blood Mononuclear Cells (PBMCs) • T cell immune responses • T cell proliferation (CFSE staining) • Th1 cytokine production (IFN-, TNF-) • CTL activity CD8+ T cells • Granzyme B & Perforin production • T cell antigen-specificity responses • Peptide pools (Env, Gag: accessory proteins) • B Cell Differentiation Assay © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 26 Chimigen® HIV Vaccine: Summary • Chimigen® HIV Multi-antigen Vaccine was designed & cloned • Prophylactic/Therapeutic applications • Vaccine • Produced in Sf9 insect cells & purified • Immune response ex vivo, using human DC/T cells • Chimigen® HIV Vaccine, in human DC/T cell assays ex vivo • Binds to immature DCs and induces both T cell and B cell responses • Induces • Activation and proliferation of CD8+ and CD4+ T cells • Production of IFN-γ and TNF-α in CD8+ and CD4+ T cells • Production of GrB+, Pfn+ CD8+ and CD4+ T cells • T cell responses are HIV antigen-specific • Production of antigen-specific antibodies (IgM) • Chimigen® HIV Multi-antigen Vaccine shows excellent potential for further development © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 27 Chimigen® HIV Vaccine Immune Responses, in vivo, (Rats) •Evaluation of antigen-specific T and B cell responses following vaccination •IFN-γ, IL-4, TNF-α secretion •Antigen-specific antibodies •Immune response specificity for recombinant Gag, Env, Tat,-Rev-Vpr-Vpu (TRVV), and murine IgG1 Fc (mTBD) Study carried out at VIDO/Intervac (University of Saskatchewan) © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 28 Chimigen® HIV Vaccine Study Design Vehicle Chimigen® HIV Vaccine (5, 10, 20 μg) • Evaluate immunogenicity of the Chimigen® HIV Vaccine • Female Sprague Dawley rats (3-4 weeks) divided into groups of 6 • Animals vaccinated subcutaneously three times (0, 4, 8 weeks) • Chimigen® Vaccine dose: 5, 10, or 20 μg • Serum was sampled every two weeks to assay for antigen-specific antibody responses to individual HIV antigens and the mTBD 3x N=6 Antibody response Cell-mediated • Immune response Splenocytes were isolated after the third immunization to assay for antigen-specific T cell responses © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 29 Chimigen® HIV Vaccine: Summary • Chimigen® HIV Vaccine is immunogenic; most of the animals responded • Chimigen® HIV Vaccine is capable of inducing antibody response • Response is specific for the vaccine component antigens • Moderate to significant response following the third immunization • Higher antigen-specific IgG2a antibody titres relative to IgG1; indicative of a Th1 immune response – Th1 immune response was vaccine-dose-dependent – Specific for Vaccine component antigens – Moderate to significant response following the third immunization • Effective at low doses • No added adjuvant • Chimigen® HIV Vaccine is very effective in inducing systemic cellmediated immune response © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 30 Chimigen® Influenza/Pandemic Vaccines New Effective Vaccines- Major Unmet Medical Need 6xHis 6xHis Influenza H5(H1)-M2 IRD Influenza H5(H1)-M2) CHO CHO Peptide Linker Portion of CH1 S-S Hinge Region Generates B Cell and T cell Immunity S-S S S CHO CH2 S S CCH2 H2 Xenotypic Murine Fc CHO TBD SS SS CH3 CH3 Peptide Chimigen® Pan-Influenza Vaccine M2 (Universal): HA (1-9) can be produced, stored as Baculovirus stock © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 31 Chimigen® WEEV Vaccine No Approved Vaccine Currently Available 6xHis Alphaviruses – Viral Encephalitis 6xHis WEEV E1-E2 WEEV E1-E2 • Single stranded RNA viruses: Venezuelan Equine CHO IRD CHO Peptide Linker Encephalitis (VEEV), Western Equine Encephalitis Portion of CH1 S-S Hinge Region S-S (WEEV), Eastern Equine Encephalitis (EEEV) SS • Viruses can be aerosolized and are highly infectious CHO CH2 SS CHO CCH2 H2 Xenotypic Fc • No definitive anti-viral treatment TBD SS • VEEV and WEEV have been successfully weaponized • Current Vaccine development focused on live, attenuated viruses • Significant side effects SS CH3 CH3 Peptide Showed efficacy in challenge model at DRDC-Suffield • Potential to mutate into virulent strains • Conventional Adjuvant Vaccines not showing promise • Applied for NIH (BARDA) grant, 2007- 2008 • US $14.8 mil. (3+1 Years) • Received high technical merit ranking (109), possible to reapply • Anthrax priority (92 & 121) © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 32 Chimigen® WEEV Vaccines Chimigen® WEEV E2 Vaccine Chimigen® WEEV E2-E11-200Vaccine Akshaya Bio Inc. …..…………..Our focus is on vaccines Chimigen® WEEV Vaccine: Results Summary • Chimigen® E2 and Chimigen® E2-E11-200 Vaccines (WEEV)have been produced • Chimigen® WEEV vaccines bound to DC • • Chimigen ® E2 Vaccine used to immunize mice • • Bound to CD32 & CD 206 Immunized mice generated a strong antibody response • IgG1 > IgG2a • Antibody response was Th2 biased • Modest T cell proliferation and IFN-γ production was observed Chimigen ® WEEV E2 and E2-E11-200 Vaccines used to immunize mice • Mice were subsequently challenged with live-WEEV • Chimigen ® WEEV vaccines shifted survival curve in a subset of the animals • There was one survivor in the Chimigen ® E2-E11-200 vaccinated mice • The vaccine showed protection against WEEV challenge • This shows early proof of concept for the Chimigen® WEEV Vaccine 34 Akshaya Bio Inc. …..…………..Our focus is on vaccines Chimigen® Ebolavirus Vaccine Ebolavirus (EBOV) • Filovirus • BSL 4 pathogen (WHO), Category A (CDC) • 19kB linear negative-sense RNA genome – Encodes 7 genes • Five species: – Cote d’Ivoire EBOV (ICEBOV) – Reston EBOV (REBOV) – Bundibugyo EBOV (BEBOV) – Sudan EBOV (SEBOV) – Zaire EBOV (ZEBOV) © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 35 Chimigen® Ebolavirus Vaccine Multiple Ebola Virus Antigen Targets 6xHis 6xHis Ebola antigens GP-NP-VP40 Ebola antigens GP-NP-VP40 CHO IRD CHO Peptide Linker Portion of CH1 Hinge Region S-S S-S S S Xenotypic (Murine)Fc CHO CHO CH2 S S CCH2 H2 TBD SS CH3 SS CH3 Predicted to Induce Humoral and Cellular Immune Responses © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 36 Cancer Vaccine Program: Early Discovery Rationale MUC1 5xTandem Repeat Chimigen® Vaccine designs have demonstrated ability to, • Target dendritic cells • Produce functional CTLs • Overcome immune tolerance • Overcome immune anergy • Overcome antigenemia MUC1 5xTandem Repeat CHO IRD CHO Peptide Linker Portion of CH1 S-S Hinge Region S-S SS CHO CH2 S S CCH2 H2 Xenotypic Murine Fc CHO TBD SS SS CH3 CH3 Peptide MUC1 is over-expressed in a large number of carcinomas Novel immunotherapy without the individualized (Autologous) vaccine concept Antigen MUC1 Cancer Targets Under Development/ Consideration Carbonic anhydrase IX (CA9) Carcinoembryonic antigen (CEA) HER 2/neu Prostatic acid phosphatase © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. Cancers Overexpressed in multiple cancers; Breast, ovarian, colon, pancreas, rectal 75% of Cervical and colon cancers and 95% of renal cancers 65% of breast cancers, 70% of lung cancers and almost 100% of colon cancers Breast, ovarian and colorectal solid tumors Prostate Cancer …..…………..Our focus is on vaccines 37 Chimigen® MUC 1 Cancer Vaccine MUC1 MUC1 10xTandem Repeat 10xTandem Repeat CHO IRD CHO Peptide Linker Portion of CH 1 S-S Hinge Region S-S SS CHO CH 2 S S CCH2 H2 Xenotypic Murine Fc CHO TBD SS SS CH 3 CH 3 Tandem repeat sequence: ACC TCG GCC CCG GAC ACC AGG CCG GCC CCG GGC TCC ACC GCC CCC CCA GCC CAC GGT GTC T S A P D T R P A P G S T A P P A H G V © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 38 Chimigen® Prostate Cancer Vaccine PAP PAP CHO IRD CHO Peptide Linker Portion of CH 1 S -S Hinge Region S -S S S CHO CH 2 S S CHO CCH2 H2 TBD Xenotypic ( Murine ) Fc S S S S CH 3 CH 3 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 39 Chimigen® CEA Cancer Vaccine CEA CEA CHO IRD CHO Peptide Linker Portion of C H 1 S-S Hinge Region S-S S S CHO CH 2 S S CHO CCH2 H2 TBD Xenotypic ( Murine ) Fc S S S S CH 3 CH 3 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 40 Chimigen® Malaria Vaccine Initial Development Funded by Gates Foundation Award GCE 3 • leading cause of death worldwide • Endemic in 108 countries • 40% (3 billion) of world population exposed to parasite 6xHis 6xHis • Estimated 247 million cases worldwide IRD • Over 1 million deaths annually • 80% deaths occur in children under 5 (weak acquired immunity) • Pregnant women more susceptible Xenotypic TBD (Murine)Fc (pregnancy associated malaria) • Vertical transmission is a major cause of miscarriage and stillbirth Targets the parasite • No approved vaccine to prevent malaria at multiple stages of the infection • Ready for animal studies 5th Malaria antigens Malaria antigens (CSP/AMA-1/LSA120 repeats/MSP-142) (CSP/AMA-1/LSA120 repeats/MSP-142) HBV Core HBV Core CHO CHO Peptide Linker Portion of CH1 Hinge Region S-S S-S S S CHO CHO CH2 S S CCH2 H2 SS CH3 SS CH3 Peptide © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 41 Chimigen® Mtb Multi-antigen Vaccine Targets tuberculosis at various stages of the infection 6xHis 6xHis Mtb Antigens (Ag85b/ESAT6/ Rv2660c/HspX/ RpfA/RpfD) Mtb Antigens (Ag85b/ESAT6/ Rv2660c/HspX/ RpfA/RpfD) CHO IRD CHO Peptide Linker Portion of CH1 S-S Hinge Region S-S SS CHO CH2 SS CHO CCH2 H2 Xenotypic Fc TBD SS SS CH3 CH3 Peptide © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 42 Chimigen® TB Multi-antigen Vaccine Tuberculosis (TB) • • • Targets tuberculosis at various stages of the infection Caused by Mycobacterium tuberculosis 8.7 million new cases in 2011 6xHis Mtb Antigens 1.4 million deaths in 2011 (Ag85b/ESAT6/ – 430,000 people with HIV – 70,000 children – Leading killer of people with HIV • CHO CHO Portion of CH1 S-S Hinge Region S-S SS CHO CH2 S S CCH2 H2 Xenotypic Fc CHO TBD SS – Not recommended in HIV+ children SS CH3 (WHO Global Advisory Committee) CH3 Peptide © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. IRD Peptide Linker 630,000 people with MDR-TB in 2011 Current vaccine (M. bovis BCG) low efficacy Mtb Antigens (Ag85b/ESAT6/ Rv2660c/HspX/ RpfA/RpfD) Rv2660c/HspX/ RpfA/RpfD) – 150,000 deaths annually from MDR-TB – 10% of MDR-TB are EDR-TB • 6xHis …..…………..Our focus is on vaccines 43 Akshaya Bio Inc. Intellectual property Portfolio Issued Patents © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 44 Akshaya Bio Inc. CHIMERIC ANTIGENS FOR ELICITING AN IMMUNE RESPONSE Issue Expiry Country Status Application No.Filing Date Patent No. Date Date 10/365,620 13-Feb-03 05-Feb-24 United States Issued 8,029,803 04-Oct-11 Country Status United States Issued United States Issued Application No. Filing Date Patent No. 10/912,969 5-Aug-04 8,025,873 13/209,979 15-Aug-11 8,465,745 Issue Date Expiry Date 27-Sept-11 05-Feb-24 18-Jun-13 13-Feb-23 HEPATITIS B FAMILY CHIMERIC ANTIGENS FOR BREAKING HOST TOLERANCE TO FOREIGN ANTIGENS Country Germany (Europe) France (Europe) Status Issued Issued Application No. Filing Date Patent No. 602004045 6-Aug-04 169.9 166270 04761663.9 06-Aug-04 166270 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. Issue Date Expiry Date 06-Aug-24 21-May-14 06-Aug-24 21-May-14 …..…………..Our focus is on vaccines 45 Akshaya Bio Inc. HEPATITIS B FAMILY CHIMERIC ANTIGENS FOR BREAKING HOST TOLERANCE TO FOREIGN ANTIGENS Expiry Country Status Application No. Filing Date Patent No. Issue Date Date United 10/913,171 5-Aug-04 05-Feb-24 States Issued 8,007,805 30-Aug-11 China Issued 200480022 6-Aug-04 ZL 27-Apr-11 6-Aug-24 747.1 200480022 747.1 Eurasia Issued 200600390. 6-Aug-04 13070 26-Feb-10 6-Aug-24 (validated in 0 RU) India Issued 00256/MU 6-Aug-04 225281 MNP/20 UK (Europe) Issued 04761663.9 6-Aug-04 1664270 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. 5-Nov-08 6-Aug-24 21-May-14 6-Aug-24 …..…………..Our focus is on vaccines 46 Akshaya Bio Inc. HEPATITIS B FAMILY CHIMERIC ANTIGENS FOR BREAKING HOST TOLERANCE TO FOREIGN ANTIGENS Japan Issued 2006-522192 6-Aug-04 5200201 New Zealand Issued 545048 6-Aug-04 545048 22-Feb-13 6-Aug-24 8-Oct-09 6-Aug-24 Singapore Issued 200600745-4 6-Aug-04 119567 29-Aug-08 6-Aug-24 Taiwan Issued 93123603.0 6-Aug-04 I364294 21-May-12 6-Aug-24 South Africa Issued 2006/00804 6-Aug-04 2006-0804 25-Apr-07 6-Aug-24 Korea Issued 10-20067002648 6-Aug-04 10-13277194-Nov.13 6-Aug-24 Australia Issued 2012200998 6-Aug-04 2012200998 27 Feb.14 6-Aug-24 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 47 Akshaya Bio Inc. HEPATITIS C FAMILY CHIMERIC HEPATITIS C VIRUS ANTIGEN FOR ELICITING AN IMMUNE RESPONSE Singapore Issued 200802303-8 13-Oct-06 141061 29-Oct-10 13-Oct-26 South Issued 2008/04078 13-Oct-06 2008/040 30-Sep-09 13-Oct-26 Africa 78 Si RNA FAMILY ANTIGENIC COMPOSITIONS AND USE OF SAME IN THE TARGETED DELIVERY OF NUCLEIC ACIDS New Zealand 584306 29-Jan- 29-AugIssued 584306 29-Aug-08 13 28 20088011360 ZL20088011 10-Dec- 29-AugChina Issued 1.6 29-Aug-08 36016 14 28 12/67556 26-Feb- 8637477 284-MarUSA Issued USA Issued 0 10 Jan-14 29 Australia Issued 2008291604 29-Aug-08 20082916 20-Mar-14 29-Aug-28 04 © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 48 Akshaya’s Competitive Advantages Product candidates meet major unmet needs No vaccine/therapy available Re-educates the immune system Targets Dendritic Cell Receptors Natural process for uptake and presentation of antigens Most efficient and natural process of antigen delivery to DCs Efficacy Demonstrated Proof of Concept established Using immune cells from HBV Chronic Carriers HBV vaccine candidate breaks the immunological tolerance to HBV antigens No Adjuvant Eliminates majority of the adverse reactions Strong Intellectual Property Position 23 issued patents, 17 pending applications, USA & World © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 49 Chimigen® Vaccine Platform - Highlights • Versatile, Adaptable Platform • Dendritic Cell Receptor-Targeted Vaccines • Generates broad immune responses • Cellular (Class I) - critical to clear virus-infected & cancer cells • Humoral (Class II) – Abs- Helps CTLs • Proof of principle established • Chimigen® HBV Therapeutic Vaccine • Potential use for Prophylactic & Therapeutic Vaccines • Strong Intellectual Property Position • 23 Issued Patents, USA and World © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 50 Akshaya Bio Inc. Contact Information Rajan George, M.Sc, Ph.D President & Chief Scientific Officer 8223 Roper Road Edmonton Alberta, T6E 6S4 Canada Telephone 780 989 6705 Mobile 780 493 1706 email [email protected] Web www.akshayabio.com © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 51 Akshaya Bio Inc. Thank You……. © 2015 Akshaya Bio | www.akshayabio.com Akshaya Bio Inc. …..…………..Our focus is on vaccines 52