* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download spdfgh
History of quantum field theory wikipedia , lookup
Quantum state wikipedia , lookup
Renormalization group wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Canonical quantization wikipedia , lookup
Renormalization wikipedia , lookup
Identical particles wikipedia , lookup
Tight binding wikipedia , lookup
EPR paradox wikipedia , lookup
X-ray fluorescence wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical bond wikipedia , lookup
Elementary particle wikipedia , lookup
Particle in a box wikipedia , lookup
Spin (physics) wikipedia , lookup
Matter wave wikipedia , lookup
Double-slit experiment wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Wave–particle duality wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
Hydrogen atom wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
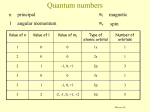
Magnetic moment Bohr magneton Magnetic moment gyromagnetic ratio (g factor) determined by details of charge distribution (elementary particle e.g. electron) (QED corrections for electron) 7-31. Assuming the electron to be a classical particle, a sphere of radius 10-15 m and a uniform mass density, use the magnitude of the spin angular momentum | S | = [s (s+1)]1/2 ħ=(3/4)1/2 ħ to compute the speed of rotation at the electron’s equator. How does your result compare with the speed of light? Magnetic moment Stern-Gerlach experiment Inhomogeneous magnetic field Stern-Gerlach experiment Inhomogeneous magnetic field Stern-Gerlach experiment Inhomogeneous magnetic field Classical picture – continuum of possible orientations Quantum mechanics– 2l +1 deflections ? Stern-Gerlach experiment Total Angular Momentum total angular momentum or If J1 is one angular momentum (orbital, spin, or a combination) and J2 is another, the resulting total angular momentum J = J1 + J2 has the value [ j ( j + 1)]1/2 ħ for its magnitude, where j can be any of the values j1 + j2, j1 + j2 - 1, . . . , | j1 - j2 | 7-34. (a) The angular momentum of the yttrium atom in the ground state is characterized by the quantum number j = 3/2. How many lines would you expect to see if you could do a Stern-Gerlach experiment with yttrium atoms? (b) How many lines would you expect to see if the beam consisted of atoms with zero spin, but l= 1? a) b) 7-37. A hydrogen atom is in the 3d state (n = 3, l = 2). (a) What are the possible values of j? (b) What are the possible values of the magnitude of the total angular momentum? (c) What are the possible z components of the total angular momentum? a) b) c) Spectroscopic Notation Single electron l s p d f g h 0 1 2 3 4 5 n K L M N O 1 2 3 4 5 Atomic state total spin n Hydrogen ground state total orbital angular momentum total angular momentum Identical Particles in Quantum Mechanics Non-interacting particles e.g. Identical Particles in Quantum Mechanics Symmetric wavefunctions –bosons (e.g photons) Antisymmetric wavefunctions –fermions (e.g electrons) Symmetric wavefunctions –bosons (e.g photons) Antisymmetric wavefunctions –fermions (e.g electrons) Pauli Exclusion Principle No more than one electron may occupy a given quantum state specified by a particular set of singleparticle quantum numbers n, l, ml ms. Ground State of Atoms He (Z=2) 1s2 – ground state (more accurate calculations to be used) He+ Energy needed to remove the first electron (first ionization) potential is 24.4 eV Ground State of Atoms l=0 m=0 ms=±1/2 2 electrons l=1 m=-1,0,1 ms=±1/2 6 electrons l=2 m=-2,-1,0,1,2 ms=±1/2 10 electrons ground state H : 1s1 He: 1s2 (filled shell n=1) last electron n=1, l=0 n=1, l=0 Li: 1s2 2s1 n=2, l=0 Be: 1s2 2s2 n=2, l=0 B : 1s2 2s2 2p1 n=2, l=1 C : 1s2 2s2 2p2 n=2, l=1 N: 1s2 2s2 2p3 n=2, l=1 O: 1s2 2s2 2p4 n=2, l=1 F: 1s2 2s2 2p5 n=2, l=1 Ne: 1s2 2s2 2p6 (filled shell n=2) n=2, l=1 Ground State of Atoms ground state last electron Na: 1s2 2s2 2p6 3s1 n=3, l=0 Mg: 1s2 2s2 2p6 3s2 n=3, l=0 Al: 1s2 2s2 2p6 3s2 3p1 n=3, l=1 Si : 1s2 2s2 2p6 3s2 3p2 n=3, l=1 P: 1s2 2s2 2p6 3s2 3p3 n=3, l=1 S: 1s2 2s2 2p6 3s2 3p4 n=3, l=1 Cl: 1s2 2s2 2p6 3s2 3p5 n=3, l=1 Ar: 1s2 2s2 2p6 3s2 3p6 n=3, l=1 K: 1s2 2s2 2p6 3s2 3p6 3d1 n=3, l=2 K: 1s2 2s2 2p6 3s2 3p6 4s1 n=4, l=0 Ground State of Atoms 2p state is almost always outside 1s electrons - sees the effective charge Zeff =+1 2s state penetrates the shielding of 1s electrons more positive charge –lower energy for 2s Ground State of Atoms Li: 1s2 2s1 Greater l smaller involves penetration effect and large energies The large penetration effect makes the energy 4s lower than 3d. 7-46. Write the ground-state electron configuration of (a) carbon, (b) oxygen, and (c) argon. C : 1s2 2s2 2p2 O: 1s2 2s2 2p4 Ar: 1s2 2s2 2p6 3s2 3p6 7-50. If the 3s electron in sodium did not penetrate the inner core, its energy would be -13.6 eV/32 = -1.51 eV. Because it does penetrate, it sees a higher effective Z and its energy is lower. Use the measured ionization potential of 5.14 V to calculate Zeff for the 3s electron in sodium. 7-41. The Lamb shift energy difference between the 22S1/2 and 22P1/2 levels in atomic hydrogen is 4.372 x 10-6 eV. (a) What is the frequency of the photon emitted in this transition? (b) What is the photon’s wavelength? (c) In what part of the electromagnetic spectrum does this transition lie? a) b)