* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download History of the Atom
Fundamental interaction wikipedia , lookup
History of quantum field theory wikipedia , lookup
Elementary particle wikipedia , lookup
Time in physics wikipedia , lookup
Anti-gravity wikipedia , lookup
Condensed matter physics wikipedia , lookup
History of subatomic physics wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Maxwell's equations wikipedia , lookup
Field (physics) wikipedia , lookup
Electromagnet wikipedia , lookup
Superconductivity wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Magnetic monopole wikipedia , lookup
Electric charge wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Electrostatics wikipedia , lookup
Lorentz force wikipedia , lookup
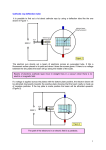
History of the Atom: Physics Chapter 27 Early work in Electricity and Magnetism Oersted: Current makes magnetic field Faraday/Henry: Magnetic fields moving make currents Maxwell: Electricity, magnetism and light are all parts of the electromagnetic field Hertz: Experiments supported Maxwell’s work James Clerk Maxwell 1831-1879 Showed that electricity and magnetism were related, and were related to atoms Predicted that accelerating charges would make waves (electromagnetic radiation) Cathode Ray Tube Experiments Glass tube with wire at each end; as much air pumped out as possible Charge passed across tube makes fluorescent glow William Crookes Tube coated with fluorescent material can be made to glow in one focused dot Rays travel in straight lines Ray carries negative charge Joseph John Thomson 1856-1940 Used a study of the cathode ray tube to determine the presence of electrons 1897 Suggested the plum pudding model of the atom and the existance of isotopes Won the Nobel Prize in Physics in 1906 J. J. Thomson’s Experiment Thomson used both a magnetic field and electric field to deflect the electrons He measured the deflection of the ray and calculated the charge:mass ratio of electrons J. J. Thomson’s Experiment Used magnetic field to show cathode rays had negative charge Used electric field to show cathode rays were particles with negative charge Used varying electric currents to determine charge to mass ratio Force caused by electric field: qE Force caused by magnetic field: Bqv When these forces are equal Bqv=qE Then v = E/B When electric field removed, particles given centripetal force by magnetic field Bqv = mv2/r Solved for mass/charge ratio: m/q = Br/v Thomson calculated m/q as 5.686 x 10-12 kg/C Evidence suggested particles very small and came from atom J. J. Thomson’s experiment Thomson calculated m/q as 5.686 x 10-12 kg/C Millikan calculated q = 1.602 x 10-19 C Can be used to calculate m: m=(5.686 x 10-12 kg/C) q m calculated as 9.109 x 10-31 kg Method can be used for any charged particle Example A beam of electrons travels an undeflected path in a cathode ray tube. E is 7.0 x 103 N/C. B is 3.5 x 10-2 T. What is the speed of the electrons as they travel through the tube? What we know: E = 7.0 x 103 N/C B=3.5 x 10-2 T Equation: v = E/B Substitute: v = (7.0 x 103N/A s) / (3.5 x 10-2 N/A m) Solve! v = 2.0 x 105 m/s Example An electron of mass 9.11 x 10-31 kg moves with a speed of 2.0 x 105 m/s across a magnetic field. The magnetic induction is 8.0 x 10-4 T. What is the radius of the circular path followed by the electrons while in the field? What we know: M = 9.11 x 10-31 kg B=8.0 x 10-4 T Equation: Bqv = mv2/r so r = (mv) / (Bq) Substitute: R = (9.11x10-31kg)(2.0x105 m/s) (8.0x10-4N/Am)(1.6x10-19As) Solve! r = 1.4 x 10-3 m v=2.0 x 105 m/s Robert A. Millikan 1858-1953 Used the 'falling drop method' to determine the charge of the electron (-1.6022 x 10-19 C) and mass of electron as 9.10 x 10-28 g Investigated photoelectric effect and spectroscopy of elements Won the Nobel Prize in Physics in 1923