* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Esterification
George S. Hammond wikipedia , lookup
Bottromycin wikipedia , lookup
Kinetic resolution wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Petasis reaction wikipedia , lookup
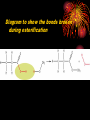
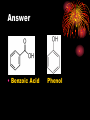
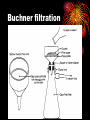
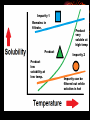
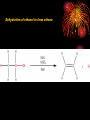
How well are you doing? Where did the class fall down on the last past paper? Esters, Equilibria and Testing for ions Esterification and Dehydration Success Criteria: Be able to • Describe the esterification of alcohols with carboxylic acids. • Produce a sample of a pure Phenyl Benzoate ester using a range of advanced chemical techniques. • Explain the process of re-crystallisation. • Extension: • Describe elimination of H2O from alcohols to form alkenes. Numeracy Starter • What is the resolution of measuring cylinder A? Numeracy Starter • What is the resolution of measuring cylinder B? • S&C why is there a temperature on the cylinder? Which measuring cyclinder would you use to measure 2cm3 of liquid? Why? TASK : Calculate the % error in each case. Numeracy Starter Answer 3 • The 500cm cylinder had a resolution of +/-2.5cm3. Therefore to measure 2cm3 the error would be (2.5/2.0)x100 = 125% • The 5cm3 measuring cylinder had a resolution of +/- 0.1cm3 Therefore to measure 2cm3 the error would be (0.1/2.0) x 100 = 5% AFL • On your post-it note write down • A/ How you would make the ester phenyl benzoate. • B/ How you would separate and purify the product. • If you don’t know say so. That is fine! Name the compound… Name the compound The esterification reaction between propanoic acid and methanol Diagram to show the bonds broken during esterification Naming esters Simple laboratory preparation of an ester Making Pentyl Ethanoate 2cm3 of Pentan-1-ol, 2cm3 of glacial ethanoic acid. Add a few drops of concentrated sulfuric acid. HAZARDOUS Separating the ester Boiling points 118oC 65oC Miscible with water? Yes Yes 57oC No 100oC Yes Suggest two ways that you could separate the ester from the reaction mixture. Pour your reaction mixture into water Ester floats on the water as it is less dense and immiscible Sulfuric acid, ethanoic acid and pentan-1-ol are soluble in water Mini Plenary • How do you make an ester? • What is the catalyst used? • How do you separate the ester from the reaction mixture? • Name the ester made from ethanol and propanoic acid. Homework • Describe and explain the formation of phenyl benzoate from benzyl chloride and phenol including a detailed explanation of recrystallization. Spaced Learning Activity • You have four minutes to use the modelling putty to make a model of a bunch of grapes. Esters in grapes • Pembrokeshire has a vineyard which produces wines with fine flavours produced by esters. • Research has identified 11 different esters in grape juice, but wine can have up to 83; this affects the wine’s flavour and complexity in proportion to their presence. Esters are formed when an acid reacts with alcohol, eliminating a water molecule in the process. Ethyl esters have a strong influence on a wine’s aroma here are three examples; • ethyl hexanoate, fruity, strawberry, green apple and anise • ethyl octanoate, with sweet, fruity, ripe fruit, burned and beer characteristics • ethyl decanoate, which imparts an oily, fruity and floral character. Sweet-smelling esters Explain how you would make Octyl Ethanoate The esterification reaction is both slow and reversible. The equation for the reaction between ethanoic acid and ethanol is: 1180C 780C 540C 1000C Q1 What does reversible mean? DH of the forward reaction is -20kJ.mol-1 Q2 How can we favour the forward reaction and make more Ester? Q3 Why do we warm the reaction mixture? Starter Synthesis of PhenylBenzoate • Draw the two compounds that you would need to react together to form phenylbenzoate. Answer • Benzoic Acid Phenol How to get around reversible reactions.. By using a derivative of carboxylic acids called an acid chloride in the presence of sodium ions we minimise the back reaction from taking place to gain a better yield of the ester. We also do not need the acid catalyst if we do this. Risk Assessment • Hazard- Phenol is corrosive and causes burns to the skin. • Risk – Phenol getting on skin during weighing/ handling. • Wear gloves, lab coats and safety glasses. Inform teacher immediately if phenol gets on your skin. Method 1/ Weigh 1.0g of Phenol into a small conical flask. HAZARD 2/Add 18cm3 of 1M NaOH(aq) and bung the flask. 3/ HAZARD In a fume cupboard add 2cm3 of Benzoyl chloride in small quantities at a time. 4/ Fit a bung and shake vigorously with occasional cooling under the tap or in ice water. Releasing the gas formed into the fume cupboard every few minutes. 5/ After 15 minutes the solid benzoate separates out: the solution should be alkaline at the end of the reaction; if not alkaline, or if oily, add a solid pellet of sodium hydroxide and shake again. 6/ Buchner filtrate the phenyl benzoate and wash thoroughly with ice cold water. and re-crystallise from ethanol (NO FLAMES!). Buchner filtration Impurity 1 Remains in filtrate. Product Product low solubility at low temp. Product very soluble at high temp Impurity 2 Impurity can be filtered out while solution is hot Numeracy starter If a saturated solution of phenylbenzoate in ethanol was cooled from 55oC to 20oC what mass of the ester would be precipitated? Recrystalisation to purify your product. • What is your product contaminated with? • How can we remove this? Recrystallisation • → Solvent added (clear) to compound and soluble impurities (orange) → Solvent heated to give saturated compound solution (orange) with impurities in solution → solution (orange) allowed to cool over time to give crystals of product (orange) and a solution of impurities (pale-orange). Method 1/ Warm 2/3 of a boiling tube of ethanol to 70oC. 2/ Place the impure Phenyl Benzoate crystals into a second boiling tube and place the tube in the water bath. 3/ Add the minimum quantity of hot ethanol to the crystals to get them to dissolve. Work with a glass rod. 4/ When all the crystals have dissolved take the tube out of the water bath and place in a test tube rack. 5/ Observe with care DO NOT DISTURB. 6/ Note your observations. 7/ Buchner filtrate your crystals when cool. Phenyl Benzoate crystals Dehydration of alcohols to form Alkenes. Dehydration of cyclohexanol to form cyclohexene Dehydration of ethanol to form ethene © Pearson Education Ltd 2008 This document may have been altered from the original Extension questions