* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Kinetic resolution wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
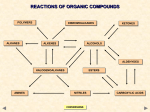
A Reactions Review 1. 2. 3. 4. 5. 6. FREE RADICAL SUBSTITUTION POLYMERIZATION ELECTROPHILIC ADDITION ( types) A) Hydration (add water, HOH) B) Halogenation (add H2) C) Bromination (add Br2) D) Hydrohalogenation (add HCl, HBr,HI (acids) ELIMINATION (Dehydrohalogenation) NUCLEOPHILIC SUBSTITUTION (add :OH-,:CN-,H2O, 2:NH3) OXIDATION A) 10 ALCOHOLS TO ALDEHYDES THEN TO CARBOXYLIC ACIDS B) 20 ALCOHOLS TO KETONES REACTIONS OF ORGANIC COMPOUNDS POLYMERS ALKANES DIBROMOALKANES ALKENES KETONES ALCOHOLS ALDEHYDES HALOGENOALKANES AMINES NITRILES CONVERSIONS CARBOXYLIC ACIDS 14 REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES KETONES P F C D ALKANES ALKENES E ALCOHOLS M A B N L ALDEHYDES G HALOGENOALKANES O H AMINES I NITRILES CARBOXYLIC ACIDS A Initiation Free radical substitution Cl2 Propagation Cl• + CH4 Cl2 + CH3• Termination Cl• + Cl• Cl• + CH3• CH3• + CH3• ——> 2Cl• radicals created ——> CH3• + HCl ——> CH3Cl + Cl• radicals used and then re-generated ——> ——> ——> radicals removed Cl2 CH3Cl C2H6 Summary Due to the lack of reactivity of alkanes you need a very reactive species to persuade them to react. This is done by shining UV light on the mixture (heat could be used) CONVERSIONS ELECTROPHILIC ADDITION OF HBr B (Hydrohalogenation) Reagent Hydrogen bromide... it is electrophilic as the H is slightly positive Condition Room temperature. Equation C2H4(g) + HBr(g) ———> C2H5Br(l) bromoethane Mechanism Step 1: As the HBr nears the alkene, the Pi bond breaks The δ positive H joins a carbon (hydrogenation) The :Br ion forms. Also a carbocation (positively charged carbon species) is formed. Step 2 The bromide ion (halogen) is a nucleophile and attacks the carbocation. Br across the double bond. CONVERSIONS C ELECTROPHILIC ADDITION OF Br2 Reagent Bromine. (Neat liquid or dissolved in tetrachloromethane, CCl4 ) Conditions Room temperature. No catalyst or UV light required! Equation C2H4(g) + Br2(l) ——> CH2BrCH2Br(l) 1,2 - dibromoethane Mechanism It is surprising that bromine should act as an electrophile as it is non-polar. CONVERSIONS D ELECTROPHILIC ADDITION with Water (or HYDRATION) Reagent steam Catalyst Sand coated with phosphoric acid (BOTH WAYS) Product alcohol OR Ethene (depending on direction with Chatelier's principle) H3PO4, SiO2 Equation C2H4(g) + H2O(g) C2H5OH(g) ethanol ⍙H = -45 KJ/mo So it is no surprise that the mechanism for hydration of alkenes is identical to that of dehydration of alcohols, but in the reverse order of steps. D and M are the same mechanism, in reverse CONVERSIONS M ELIMINATION OF WATER (DEHYDRATION) Reagent/catalyst phosphoric acid (H3PO4) conc. sulphuric acid (H2SO4) or conc. Conditions reflux at 180°C Equation e.g. C2H5OH(l) CH2 = CH2(g) + H2O(l) So it is no surprise that the mechanism for hydration of alkenes is identical (as it is a REVERSABLE reaction) to that of dehydration of alcohols, but in the reverse order of steps. D and M are the same mechanism, in reverse Mechanism CONVERSIONS ELECTROPHILIC ADDITION OF H2 E or HYDROGENATION Reagent Conditions Product Equation hydrogen nickel catalyst - finely divided alkanes C2H4(g) + H2(g) ———> C2H6(g) ethane Ni Use margarine manufacture (Hydrogenated fats for spreading on bread) CONVERSIONS POLYMERISATION OF ALKENES F ETHENE POLY(ETHENE) PROPENE POLY(PROPENE) CHLOROETHENE POLY(CHLOROETHENE) POLYVINYLCHLORIDE TETRAFLUOROETHENE PVC POLY(TETRAFLUOROETHENE) PTFE CONVERSIONS “Teflon” G NUCLEOPHILIC SUBSTITUTION AQUEOUS SODIUM HYDROXIDE Reagent Conditions Product Nucleophile Equation Aqueous* KOH or NaOH Reflux in aqueous solution Alcohol hydroxide ion OH¯(aq) (SOLVENT IS IMPORTANT) e.g. C2H5Br(l) + NaOH(aq) ———> C2H5OH(l) + NaBr(aq) Mechanism * WARNING It is important to quote the solvent when answering questions. Elimination takes place when ethanol is the solvent The reaction (and the one with water) is known as HYDROLYSIS CONVERSIONS NUCLEOPHILIC SUBSTITUTION H AMMONIA Reagent Conditions Product Nucleophile Aqueous, alcoholic ammonia (in EXCESS) Reflux in aqueous, alcoholic solution under pressure Amine Ammonia (NH3) e.g. C2H5Br + 2NH3 (aq / alc) ——> C2H5NH2 + NH4Br Equation (i) (ii) C2H5Br + NH3 (aq / alc) ——> C2H5NH2 + HBr HBr + NH3 (aq / alc) ——> NH4Br Mechanism Notes The equation shows two ammonia molecules. The second one ensures that a salt is not formed. Excess ammonia is used to prevent further substitution (SEE NEXT SLIDE) CONVERSIONS NUCLEOPHILIC SUBSTITUTION I POTASSIUM CYANIDE Reagent Conditions Product Nucleophile Aqueous, alcoholic potassium (or sodium) cyanide Reflux in aqueous , alcoholic solution Nitrile (cyanide) cyanide ion (CN¯) Equation e.g. C2H5Br + KCN (aq/alc) ———> C2H5CN + KBr(aq) Mechanism Importance it extends the carbon chain by one carbon atom the CN group can then be converted to carboxylic acids or amines. K Hydrolysis C2H5CN + CONVERSIONS 2H2O ——> C2H5COOH + NH3 ELIMINATION L Reagent Alcoholic sodium (or potassium) hydroxide Conditions Reflux in alcoholic solution Product Alkene Mechanism Elimination Equation C3H7Br + NaOH(alc) ———> C3H6 + H2O + NaBr Mechanism the OH¯ ion acts as a base and picks up a proton the proton comes from a C atom next to the one bonded to the halogen the electron pair moves to form a second bond between the carbon atoms the halogen is displaced; overall there is ELIMINATION of HBr. With unsymmetrical halogenoalkanes, a mixture of products may be formed. CONVERSIONS OXIDATION OF PRIMARY ALCOHOLS N Primary alcohols are easily oxidised to aldehydes e.g. ———> CH3CH2OH(l) + [O] CH3CHO(l) + H2O(l) it is essential to distil off the aldehyde before it gets oxidised to the acid CH3CHO(l) + [O] ———> OXIDATION TO ALDEHYDES DISTILLATION CH3COOH(l) OXIDATION TO CARBOXYLIC ACIDS REFLUX Aldehyde has a lower boiling point so distils off before being oxidised further Aldehyde condenses back into the mixture and gets oxidised to the acid CONVERSIONS O OXIDATION OF ALDEHYDES Aldehydes are easily oxidised to carboxylic acids e.g. CH3CHO(l) + [O] • • • • ———> CH3COOH(l) one way to tell an aldehyde from a ketone is to see how it reacts to mild oxidation ALDEHYES are EASILY OXIDISED KETONES are RESISTANT TO MILD OXIDATION reagents include TOLLENS’ REAGENT and FEHLING’S SOLUTION TOLLENS’ REAGENT Reagent ammoniacal silver nitrate solution Observation a silver mirror is formed on the inside of the test tube Products silver + carboxylic acid Equation Ag+ + e- ——> Ag FEHLING’S SOLUTION Reagent a solution of a copper(II) complex Observation a red precipitate forms in the blue solution Products copper(I) oxide + carboxylic acid Equation Cu2+ + e- ——> Cu+ CONVERSIONS OXIDATION OF SECONDARY ALCOHOLS P Secondary alcohols are easily oxidised to ketones e.g. CH3CHOHCH3(l) + [O] ———> CH3COCH3(l) + H2O(l) The alcohol is refluxed with acidified K2Cr2O7. However, on prolonged treatment with a powerful oxidising agent they can be further oxidised to a mixture of acids with fewer carbon atoms than the original alcohol. CONVERSIONS