* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 18.1 Raman Scattering
Optical rogue waves wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Photomultiplier wikipedia , lookup
Spectral density wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Atmospheric optics wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Silicon photonics wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Upconverting nanoparticles wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Gaseous detection device wikipedia , lookup
Johan Sebastiaan Ploem wikipedia , lookup
Photonic laser thruster wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Optical amplifier wikipedia , lookup
Nonlinear optics wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Chemical imaging wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
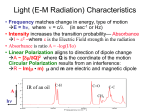
18.1 Raman Scattering • an energy level diagram shows four types of scattering processes • Rayleigh scatter is due to microscopic refractive index changes, while Raman scatter is due to time-dependent changes in polarizability • scattering cross-sections are used to compare signal strengths from Rayleigh, Raman and fluorescence • several Raman spectra are examined that show interesting features 18.1 : 1/12 Energy Level Diagram for Scattering The two horizontal dashed lines are called virtual energy levels. They are called virtual because it does not correspond to any quantum state of the molecule. The energy of a virtual transition is equal to the energy of the photon. The scatter photon appears within 10-15 seconds of excitation. S1* resonance Raman Rayleigh Raman antiStokes Raman There are two general classes of scattering events - elastic and inelastic. Elastic scatter, such as Rayleigh, emits the same energy (or S0 wavelength) photon as the exciting photon. Inelastic scatter, such as Raman, absorbs sufficient energy to promote a rotational or vibrational transition. The remaining energy is scattered as a lower energy photon. Scattered light has a directional probability with the shape of a torus. The torus axis is in the direction of the electric vector. 18.1 : 2/12 v=2 v=1 v=0 Polarization of Bound Charge When the average charge separation in a material is changed from its equilibrium value, the electrons are said to be polarized. The magnitude of the polarization vector, P, is given by P = NqX electron cloud *+ ! where N is the number of electrons, q is the electron charge and X is a spatial vector. Polarization can be caused by the electric vector of light, E, P = ε0χE where ε0 is the permittivity of free space and χ is the electric susceptibility. χ has values from 0 to ∞, and varies with density. A vacuum has χ = 0, while a metal will have a very large value. Because the electric vector of light is oscillatory, the polarized electrons will oscillate at the optical frequency. The optically induced polarization will relax within a few cycles and emit a photon due to the moving charge. 18.1 : 3/12 *+ Rayleigh Scatter When light travels through a vacuum it cannot be seen unless it is impinging on the detector (eye). This is because the waves destructively interfere in all directions except the direction of travel. When light travels through a transparent material the electric vector polarizes the electron cloud. The extent of polarization depends upon the susceptibility. In turn, the susceptibility depends upon the number of the polarizable electrons contained with a cube of volume λ3. For materials where the number of electrons varies within that volume due to molecular motion, the susceptibility varies. This variation allows a small amount of constructive interference in other directions and is the origin of Rayleigh scatter. Gases have the largest amount of Rayleigh scatter because their number densities vary ~0.05% at 500 nm and STP. Because liquids are more dense, the variation in the number of molecules per λ3 is only ~0.0016%, and scatter is less. Pure crystalline solids have very little Rayleigh scatter, which arises only because of lattice vibrations. 18.1 : 4/12 Raman Scatter When the polarizable electrons are within a molecule, vibrations add time dependent components to the polarization. P = ε 0 ⎡⎣ χ + Δχ1 cos ( 2πν1t ) + … + Δχ 3 N − 6 cos ( 2πν 3 N − 6t ) ⎤⎦ E0 cos ( 2πν Lt ) In the above equation, each numerical subscript refers to one of the 3N - 6 normal modes. The Δχ are much smaller than χ. Consider the polarization due to one of the vibrations, ν1. P1 = ε 0Δχ1 cos ( 2πν1t ) E0 cos ( 2πν Lt ) = ε 0Δχ1E0 2 ⎡ cos ( 2π [ν L + ν 1 ] t ) + cos ( 2π [ν L −ν 1 ] t ) ⎤ ⎣ ⎦ The cos(2π[νL - ν1]t) term is the Stoke's Raman band, while the cos(2π[νL + ν1]t) term is the anti-Stoke's Raman band. The antiStoke's band will be temperature dependent, just like an infrared hot band. In order to be Raman active, a vibration must change the polarizability of the electron cloud. All Raman calculations should be done in cm-1, not nanometers! 18.1 : 5/12 Pre-Resonance Raman Scatter When the laser frequency does not quite reach the electronic energy level, νL < νe, the intensity of Raman scatter is enhanced. The frequency dependence of the enhancement is given by the Albrecht-Hutley equation, ( ⎡ 2 2 2 − + ν ν ν ν ( ) L vib e L ⎢ IR ∝ ⎢ 2 ⎢ ν e2 − ν L2 ⎣ ( ) ) ⎤ ⎥ ⎥ ⎥ ⎦ 2 where νL is the laser frequency, νvib is the vibrational frequency, and νe is the electronic transition frequency. As νL approaches νe, the denominator goes to zero and the intensity increases rapidly. When νL << νe, the equation can be simplified to a form showing that Raman intensities are proportional to the fourth power of the laser. 4 ν L − ν vib ) ( IR ∝ 4 νe 18.1 : 6/12 Emission Cross-Sections Both fluorescence and scattering processes have cross-sections which include the solid angle. A solid angle has units of steradians, where a sphere of unit radius is defined as having 4π steradians. A strong absorption band has a cross-section, σa = ~10-16 cm2. A corresponding fluorescence cross-section takes into account the quantum yield and emission into 4π steradians. σf = φ f σa 4π For φf = 1, the fluorescence cross-section corresponding to the above absorption value would be 8×10-18 cm2 sr-1. A typical cross-section for Rayleigh scatter is 10-28 cm2 sr-1. Because Rayleigh depends upon the fourth power of the frequency, the value will vary a bit. A typical cross-section for Raman scatter is 10-30 cm2 sr-1. Again the exact value will depend upon the excitation frequency and distance from the absorption band. 18.1 : 7/12 Scatter Signal Strength An equation can be written to estimate and compare signal strengths for fluorescence, Rayleigh, and Raman scatter, I emission = I laserσ lN ΩF ( λ ) Δλ E ( λ ) D ( λ ) where σ is the cross-section, l the pathlength, N the number density, Ω is the lens solid angle, F(λ)Δλ the fraction of the spectrum examined, E(λ) the efficiency of the detection optics excluding the lens, and D(λ) is the detector efficiency. The term F(λ)Δλ varies for the three signals. Rayleigh scatter has a width of ~5 cm-1, Raman ~50 cm-1, and fluorescence ~5,000 cm-1. If F(λ)Δλ = 1 for Rayleigh it is ~0.1 for Raman and ~0.001 for fluorescence. The number density for water is 3.3×1022 cm-3. Using the appropriate cross-section and leaving all other terms constant gives the following relative signal strengths. I rayleigh = 3.3 × 10−6 I laser l ΩE ( λ ) D ( λ ) I raman = 3.3 × 10−9 I laser l ΩE ( λ ) D ( λ ) 18.1 : 8/12 Comparison of Scatter and Fluorescence What concentration of fluorophore will give a signal strength equal to that for solvent Raman? Use the fluorescence cross-section and F(λ)Δλ information from the last two slides. I fluor = 8 × 10−21 NI laser lΩE ( λ ) D ( λ ) 3.3 × 10−9 I laser lΩE ( λ ) D ( λ ) = 8 × 10−21 NIlaser lΩE ( λ ) D ( λ ) N= 3.3 × 10−9 8 × 10−21 = 4.1 × 1011 cm-3 This number density corresponds to 6.8×10-10 M. Conclusions: (1) In Raman spectroscopy Rayleigh scatter will be a thousand times more intense and needs to be rejected instrumentally. (2) Raman spectroscopy is difficult in the presence of a fluorophore concentration greater than nanomolar. (3) Rayleigh and Raman scatter from the solvent will make fluorescence measurements difficult below nanomolar fluorophore. 18.1: 9/12 Example Spectra (1) The large peak at 0 cm-1 is Rayleigh scatter. The peak at 1,060 cm-1 is the characteristic benzene ring vibration. The peak above 3,000 cm-1 is the C-H stretch. Compared to benzene a couple new ring vibrations have been added. The new band just under 3,000 cm-1 is the methyl C-H stretch. The low frequency band is the CH3-φ stretch. 18.1 : 10/12 Example Spectra (2) The small peak above 3,000 cm-1 is due to the alkene C-H stretch, while the stronger peak below 3,000 cm-1 is due to the alkane C-H stretch. The peak just below 1,600 cm-1 is the double bond. All of the frequencies are low because chlorine is so heavy. Note the strength of the ~200 cm-1 antiStokes band compared to the Stokes band. 18.1 : 11/12 Example Spectra (3) The O-H stretch is typically about 3,600 cm-1. Water has most of its vibrational band at lower energies because of hydrogen bonding. The bandwidth is also due to hydrogen bonding. Water Raman is often misassigned as fluorescence. Note the real fluorescence interference at lower wave numbers. The Raman spectrum of motor oil dissolved in CCl4 is overwhelmed by fluorescent components of the oil. Note the nice progression of antiStokes lines. Ordinarily the instrument is not allowed to scan over the Rayleigh line!!! It wrecks the detector. 18.1 : 12/12