* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gene therapy and viral vector
Cre-Lox recombination wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Genome evolution wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Minimal genome wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Non-coding DNA wikipedia , lookup
Protein moonlighting wikipedia , lookup
DNA vaccination wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Point mutation wikipedia , lookup
Transcription factor wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
History of genetic engineering wikipedia , lookup
Genomic library wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Adeno-associated virus wikipedia , lookup
Primary transcript wikipedia , lookup
Helitron (biology) wikipedia , lookup
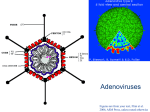
Gene therapy and viral vector Lecture 3 Viral Vectors Retrovirus Adenovirus Adeno-associated virus Herpes virus Pox virus Human foamy virus (HFV) Lentivirus Adenovirus Medium-sized (90-100nm) Icosahedral viruses Nonenveloped (without an outer lipid bilayer) Double stranded DNA Derived from Human Adenoids (tonsils) in 1953 They have a broad range of vertebrate hosts; in humans, 100 distinct adenoviral serotypes have been found to cause a wide range of illnesses, from mild respiratory infections in young children (known as the common cold) to life-threatening multiorgan disease in people with a weakened immune system. Serotype or serovar are distinct variations within a species of bacteria or viruses or among immune cells of different individuals. Different types/serotypes are associated with different conditions: respiratory disease (mainly species HAdV-B and C) conjunctivitis (HAdV-B and D) gastroenteritis (HAdV-F types 40, 41, HAdV-G type 52) obesity or adipogenesis (HAdV-A type 31, HAdV-C type 5, HAdV-D types 9, 36, 37) Structure of Adenovirus The 38 genes in the Human Adenovirus E genome are organized in 17 transcription units, each containing 1-8 coding sequences. Alternative splicing during processing of the pre-mRNAs produced by each transcription unit enable multiple different mRNAs to be produced from one transcription unit. The E1A, E1B, E2A, E2B, E3, and E4 transcription units are successively transcribed early in the viral reproductive cycle. The proteins coded for by genes within these transcription units are mostly involved in regulation of viral transcription, in replication of viral DNA, and in suppression of the host response to infection. The L1-L5 transcription units are transcribed later in the viral reproductive cycle, and code mostly for proteins that make up components of the viral capsid or are involved in assembly of the capsid. The L1-L5 transcription units are all regulated by the same promoter region and share the same transcription start site. As a result, transcription of all five late transcription units begins at the same point in the viral reproductive cycle. The functions of many adenovirus proteins are known Structural proteins include capsid proteins II (hexon), III (penton base), IIIa, IV (fiber), VI,VIII, and IX; and core proteins V,VII, X, and the terminal protein TP. Encapsidation proteins IVa2, 52K, and L1, and hexon assembly protein 100K are involved in assembly of viral capsids. The L3 protease cleaves viral precursor proteins pTP, pVI, pVII, pVIII, and IIIa to produce the mature viral proteins. Control protein E1A activates transcription of a number of viral genes as well as genes of the host cell. Control protein E1B 19K suppresses apoptosis by mimicking the action of cellular protein Bcl-2. Control protein E1B 55K binds to and inactivates the transcriptional regulator p53, thus blocking transcription of genes normally activated by p53 and contributing to the suppression of apoptosis. The functions of many adenovirus proteins are known The three proteins coded for by the E2A and E2B transcription units are all involved in replication of viral DNA. Adenovirus DNA replication begins at each end of the viral DNA, using the TP protein (rather than RNA) as a primer, so the viral DNA polymerase replicates every base of the genome. Membrane protein E3 RID-alpha and membrane protein E3 RID-beta performs a variety of molecular functions that contribute to inhibiting apoptosis. CR1 beta membrane glycoprotein modulates the host immune response. Membrane glycoprotein E3 gp19K inihibits the insertion of class I MHC proteins in the host-cell membrane, thereby preventing T-cell lymphocytes from recognizing that the host cell has been infected by a virus. Control protein E3 14.7K protects the virus from host antiviral responses. The control proteins of the E4 transcription unit are involved in regulating transcription of viral DNA. How Adenovirus enters the host cell? Entry of adenoviruses into the host cell involves two sets of interactions between the virus and the host cell. Entry into the host cell is initiated by the knob domain of the fiber protein binding to the cell receptor. The two currently established receptors are: CD46 for the group B human adenovirus serotypes and the coxsackievirus adenovirus receptor (CAR) for all other serotypes. This is followed by a secondary interaction with an integrin molecule. It is the co-receptor interaction that stimulates entry of the adenovirus. Binding to integrin results in endocytosis of the virus particle via clathrin-coated pits. Attachment to integrin stimulates cell signaling and thus induces actin polymerization resulting in entry of the virion into the host cell within an endosome. Entry in the nucleus Once the virus has successfully gained entry into the host cell, the endosome acidifies, which alters virus topology. These changes, as well as the toxic nature of the pentons, destroy the endosome, resulting in the movement of the virion into the cytoplasm. With the help of cellular microtubules the virus is transported to the nuclear pore complex, whereby the adenovirus particle disassembles. Viral DNA is subsequently released, which can enter the nucleus via the nuclear pore. After this the DNA associateswith histone molecules. Thus, viral gene expression can occur and new virus particles can be generated. Benefits Their basic biology has been studied extensively, The viral genome can accommodate large heterologous transgene insertions, They readily infect quiescent and dividing cells, They can be amplified to high titers and they have previously been shown to be relatively safe for use in humans. The family Adenoviridae consists of five genera, including genus Mastadenovirus and genus Aviadenovirus, which infect mammals and birds respectively. The adenovirus vector most commonly used for clinical trials and experimental gene therapy applications is species C adenovirus, HAdV-C5. Drawbacks Adenovirus delivered genes can be lost due to genetic instability therefore repeated doses are necessary to maintain the expression of transgene. They would not integrate into the host genome, their gene expression is too short term. Immunologic responses against adenoviruses have made their clinical application limited to a few tissues, such as liver, lung (especially for CF(Cystic Fibrosis) treatment), or localized cancer gene therapy. Drawbacks Although the risk of serious disease following natural adenovirus infection is rare and the viral genome would not integrate into the host genome, gene therapy by adenoviral vectors has caused serious bad side effects and even death of some patients. Lecture prepared from http://www.ncbi.nlm.nih.gov/pmc/articles/ PMC3507026/#!po=5.42169 http://www.genetherapynet.com/viralvector/adenoviruses.html