* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Blood clots
Drug-eluting stent wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Intravenous therapy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
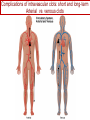
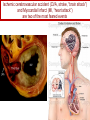
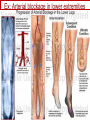
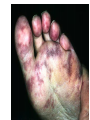
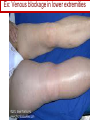
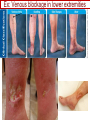
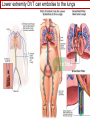
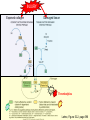
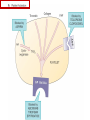
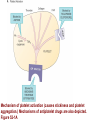
Anticoagulant, Antiplatelet, and Thrombolytic Drugs A few terms to know… Coagulation- the physiologic process by which the blood clots to form solid masses, or clots. (Over 30 types of cells and substances in blood affect clotting.) A blood clot is a thick mass of coagulated blood, platelets and other materials stuck together A thrombus (pl, thrombi) is an intravascular clot. (A thrombus can break into smaller pieces, dislodge from the location where it was initially formed.) An embolus is a blood clot, piece/globule of fatty deposit, or other object that is carried through the bloodstream. An embolism refers to the obstruction of a blood vessel by a foreign substance or a blood clot that travels through the bloodstream, lodges in a smaller blood vessel, and plugs it. Substances that can cause embolisms include: blood clots; piece/ globule of fatty deposit; clumps of bacteria, ‘vegetation’ or ‘debris’ on a heart valve; amniotic fluid; air bubble, chemicals and drugs (mainly illegal ones). Blood clots are the most common cause of embolisms. Formation of intravascular clots ► Protective when injury to blood vessel would lead to hemorrhage and blood loss ● Insults to blood vessels occur in normal physiology ► Problematic, dangerous and even life-threatening, for example: ● Certain hypercoaguable states ‒ Genetic; iatrogenic; diseases such as leukemia ● Prosthetics ‒ artificial heart valves ● Impaired mobility ‒ Post-operative bed-rest; long airplane flights ● Emergency situations ‒ Ischemic stroke; myocardial infarction; massive pulmonary embolus ● Other ►Therefore, drugs are commonly used to reduce risk of clot, or to treat a clot that has formed Physiology and Pathophysiology of Coagulation ● Thrombosis‒ may result in severe consequences, due to reduction or cessation of blood flow to a tissue by the thrombus itself, or by rupture and release of thrombi ● Arterial thrombosis• Platelet adhesion (often ruptured atherosclerotic plaque) • Release of ADP & TXA2 • Causes decreased/ absent perfusion distally -medical emergency • Depending on source of embolus, the clot may embolise to lower extremities, brain, heart, kidney, spleen, bowel ● Venous thrombosis • Stagnation of blood in lower extremities – Fibrin formation • Typically embolise to right side of heart then lungs (safety net) • Paradoxical emboli (cross from venous circulation through a septal defect between the right and left sides of the heart, thereby entering the arterial circulation) Complications of intravascular clots: short and long-term Arterial vs venous clots Ischemic cerebrovascular accident (CVA, stroke, “brain attack”) and Myocardial Infarct (MI, “heart attack”) are two of the most feared events Ex: Arterial blockage in lower extremities Ex: Venous blockage in lower extremities Ex: Venous blockage in lower extremities Lower extremity DVT can embolise to the lungs Animations of hemostasis (blood coagulation cascade) Hemostasis, Coagulation and Fibrinolysis https://www.youtube.com/watch?v=9QVTHDM90io The Coagulation Cascade http://www.allaboutbleeding.com/includes/videos/CoagCascade.mp4 Clot formation begins with tissue injury, which then activates platelets. When activated, platelets induce fibrin strand formation from platelet to platelet, thereby forming a fibrin mesh (clot) Figure 52-1B Mechanism of platelet aggregation and actions of antiplatelet drugs. Physiology of clot formation: •Stage One of Hemostasis: Formation of a “platelet plug” – Injury: Endothelial cells that line the blood vessel wall, and the sub-endothelial tissues, get injured » Components in the blood (eg platelets, clotting factors) are exposed to molecules from the injured endothelial cells and sub-endothelial tissues (eg tissue factor, collagen) » Note: Clotting factors, platelets and other molecules are inactive under normal conditions, but become activated sequentially – Platelets adhere to damaged tissue, become activated, amplify the clotting cascade, and aggregate at the site of injury – Vasoconstriction triggered by Injury to the blood vessel wall: smooth muscles in blood vessel wall immediately vasoconstrict the vessel, which reduces blood loss from the ruptured vessel Physiology of clot formation, cont’d: •Stage Two of Hemostasis: Coagulation leads to fibrin formation via ‒ Intrinsic pathway (aka contact activation pathway, turned on by contact with sub-endothelial collagen) ‒ Extrinsic pathway (aka tissue factor pathway, turned on by release of tissue factor from damaged cells) ‒ The intrinsic and extrinsic pathways have the common step of activating Factor X: this begins the “common pathway”. o Activation of factor X o Conversion of prothrombin to thrombin o Production of fibrin o Then, later, clot contraction • Hemostasis is kept under control by ANTITHROMBIN • Physiologic dissolution of clots (fibrinolysis) after tissue repair. •Release of Tissue Plasminogen Activator (t-PA) converts plasminogen to PLASMIN INJURY Exposed collagen Damaged tissue Thrombolytics Lehne, Figure 52-2, page 596 Drugs used to treat Thromboembolic Disorders Three major classifications of drugs: • Anticoagulants: −Suppress the coagulation cascade −Heparin, warfarin • Antiplatelets: −Inhibit platelet aggregation −Aspirin, clopidogrel • Thrombolytics: −Promote lysis of fibrin strands, causing dissolution of thrombi −tissue plasminogen activator ie t-PA (alteplase) 20 • Reduce the formation of fibrin • Two mechanisms of action • Inhibit the synthesis of clotting factors • Inhibit the activity of clotting factors HEPARIN AND HEPARIN DERIVATIVES Heparin (unfractionated, UFH) • Heparin (unfractionated) is not a single molecule, but a mixture of long polysaccharide chains, with molecular weights that vary from 3000 to 30,000 (units? kD?) – Active region is a unique penta-saccharide (five carbon sugar) – used for decades for prevention & treatment of thrombosis – Highly polar, cannot readily cross membranes – Mechanism: suppresses coagulation by helping antithrombin to inactivate clotting factors, primarily thrombin (factor II) & factor Xa, and thereby suppresses formation of fibrin • Particularly effective for prophylaxis of venous thromboses – BUT: variable anticoagulant effects, pharmacological properties, limited bioavailability, and highly variable anticoagulant response – Very rapid-acting anticoagulant (onset within minutes of IV administration) – Half-life only 1.5 hours, unless hepatic or renal disease – Hepatic metabolism, renal excretion 23 Heparin (Unfractionated, UFH) • Therapeutic uses – Preferred anticoagulant during pregnancy and when rapid anticoagulation is required • Does not enter breast milk – Pulmonary embolism (PE) – Ischemic stroke, evolving (t-PA may be preferred if given within 2 hours of symptoms) – Massive deep vein thrombosis (DVT) – For anticoagulation during extracorporeal procedures when blood flows through machine • Open heart surgery • Renal dialysis – Low-dose therapy postoperatively (DVT prophy) – Disseminated intravascular coagulation (DIC) – Adjunct to thrombolytic therapy (eg MI) 24 Heparin (Unfractionated, UFH): Units, Route, & Preparations • Heparin is dosed in units • Administered only by IV or subcut IV administration- commonly used for pts needing immediate anticoagulation IV: Typically a “loading” dose based on weight (aka “bolus”) is administered followed by continuous infusion. – INTENSIVE LAB MONITORING is required – PT/INR is checked to evaluate degree of anticoagulation induced by therapy with heparin » Blood draw is checked 4 – 6 hours after the bolus dose is administered. » Rate of continuous infusion is adjusted up or down depending on the lab results. – Labs are drawn on continual periodic basis per hospital protocol until heparin is DC’d. Dose is adjusted based on lab results – Heparin sometimes administered by intermittent infusion (uncommon) SQ administration; typically used for short-term, low-dose prophylactic tx. Monitoring labs aren’t usually required. (BID or TID) • Several formulations and concentrations available – Dilute vs concentrated; single dose vs multi-dose vials – Read labels carefully. – Check IV bag and infusion pump carefully. aPTT lab is used to monitor anticoagulation with heparin Activated partial thromboplastin time (aPTT) (Lehne, p 601) is used to determine the degree of anticoagulation induced by heparin therapy Normal healthy people not on anticoagulation therapy have an aPTT of about 40 seconds People on heparin for anticoagulation typically have a goal of aPTT in the range of 40 – 60 seconds Labs must be checked per hospital protocol (or per individual prescriber) Dose must be adjusted based on the lab results! Typically drawn every 4 – 6 hours in initial therapy If aPTT is too low, then heparin infusion needs to be increased per hospital protocol If aPTT is too high, then heparin infusion needs to be reduced per hospital protocol Usually blood draw is taken from a different extremity than the arm into which the IV heparin is infusing Heparin (Unfractionated, UFH) • Adverse effects- reduce risk of bleeding by screening pts for risk factors, monitor closely and avoid antiplatelet drugs – Hemorrhage – Heparin-induced thrombocytopenia (HIT) • antibodies form against heparin-platelet protein complexes • Increases thrombotic events • Uses up all the platelets (↓platelet level) • Is an absolute contraindication to giving heparin again – Hypersensitivity reactions commercial preparations derived from animal sources • Contraindicated – Thrombocytopenia – Uncontrollable bleeding – During and immediately after surgery of the eye, brain, or spinal cord Nursing Implications r/t Monitoring for, and Teaching, S/S of Bleeding • • • • • • • ↓BP, ↑Heart Rate Headache, faintness Petechiae, bruises, hematoma Red or black stools Cloudy or discolored urine Pelvic pain (possible ovarian hemorrhage) Lumbar pain (possible adrenal hemorrhage) 28 Nursing Implications r/t Minimizing the Risk of Bleedingteach to patient/family prn • Screen for risk factors • Follow requirements for therapeutic drug monitoring • Beware drug or food interactions. Monitor blood levels carefully any time a new drug is added, or a current drug is discontinued from the drug regimen. • Minimize physical manipulation of the patient • Minimize invasive procedures (eg foley catheter) • Avoid subcut and IM injections • Minimize concurrent use of anticoagulants – for example, heparin, warfarin, dabigatran) • Minimize concurrent use of antiplatelet drugs – for example, aspirin, clopidogrel • Use in caution in those with CPR or surgery within previous 3 weeks • Avoid noncompressable vascular puncture sites • Wear identification for emergency personnel 29 Question- which lab is used to monitor effects of heparin? A patient is receiving an intravenous infusion of heparin to treat a pulmonary embolism. What laboratory value will the nurse monitor to evaluate treatment with this medication? A. B. C. D. Activated partial thromboplastin time (aPTT) Prothrombin time (PT) Platelet count Hemoglobin and hematocrit 30 Question: antidote for heparin overdose? What is the antidote for heparin? A. B. C. D. Ferrous sulfate Atropine sulfate Protamine sulfate Magnesium sulfate 31 Low-Molecular-Weight Heparins • Heparin preparations composed of molecules that are shorter than those found in unfractionated heparin • LMWH as effective as UFH • Mechanism: inactivates Factor Xa – not effective to inactivate thrombin • Administered subcut • Dosage based on body weight Advantages: fixed dose, no lab, highly predictable plasma levels Cost: considerably more expensive Does not require labs/ monitoring; can be given at home Pharmacokinetics Higher bioavailability Longer half-life- once or twice a day dosing • Antidote for toxicity: Protamine sulfate • Low-Molecular-Weight Heparins Adverse effects – Bleeding (but less than with unfractionated heparin) – Heparin-induced thrombocytopenia (HIT) – Severe neurologic injury for patients undergoing spinal puncture or spinal epidural anesthesia • Therapeutic uses – First-line therapy for prevention and tx of DVT – Prevention of DVT following surgery • abdomen; hip or knee replacement – Off label use for DVT prophylaxis • in multi-trauma, or spinal surgery – Prevention of ischemic complications in pts with unstable angina, non Q-wave MI, and ST-elevation MI (STEMI) Three on market: Enoxaparin (Lovenox)- used in >80% of hospitals Dalteparin (Fragmin) Tinzaparin (Innohep) All anticoagulants • Pose a risk of spinal or epidural hematoma in patients undergoing spinal puncture or spinal/ epidural anesthesia • Pressure of hematoma on spinal cord can result in prolonged or permanent paralysis • See Lehne text, p 599 RECOMMENDATION: Health care professionals and institutions involved in performing spinal/epidural anesthesia or spinal punctures should determine, as part of a pre-procedure checklist, whether a patient is receiving anticoagulants and identify the appropriate timing of enoxaparin dosing in relation to catheter placement or removal. To reduce the potential risk of bleeding, consider both the dose and the elimination half-life of the anticoagulant: For enoxaparin, placement or removal of a spinal catheter should be delayed for at least 12 hours after administration of prophylactic doses such as those used for prevention of deep vein thrombosis. Longer delays (24 hours) are appropriate to consider for patients receiving higher therapeutic doses of enoxaparin (1 mg/kg twice daily or 1.5 mg/kg once daily). A postprocedure dose of enoxaparin should usually be given no sooner than 4 hours after catheter removal. In all cases, a benefit-risk assessment should consider both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors. Medwatch 11/2013 WARFARIN Warfarin, the first oral anticoagulant • Originally discovered when a farmer observed that his cattle began bleeding after ingesting spoiled clover silage – Deemed too risky for humans used as rat poison – A failed suicide attempt with large dose brought renewed clinical interest • Used to prevent thrombosis • Mechanism: suppresses coagulation by decreasing production of four clotting factors (VII, IX, X and prothrombin, all of which are dependent on vitamin K) • PK: only administered orally, well absorbed; 99% bound to albumin; unbound warfarin readily crosses into placenta and breast milk; hepatic metabolism by CYP2C9; metabolites excreted in urine & feces • Clinical considerations –Initial onset 6 – 12 hours after first dose; peak effect takes several days to develop. (Not useful in emergencies) –Effect persists 2 – 5 days after discontinuing –Drug therapy typically initiated with heparin (which has immediate onset) until warfarin is at therapeutic levels –Warfarin may need to be temporarily DC’d for elective surgery (Heparin is re-instituted during interim until safe to go back on warfarin) 37 Warfarin, the first oral anticoagulant • Lab monitoring ESSENTIAL! – Never does a person “not need” monitoring, although frequency of labs are somewhat individualized – Prothrombin time (PT)/ International normalized ratio (INR) ‒ Normal PT/INR (in an uncoagulated healthy person) is 1.0 ‒ When patients are on warfarin, the therapeutic “Goal PT/INR” is somewhat individualized and depends on condition being treated ‒ Most pts on warfarin have an anticoagulation PT/INR goal between 2.0 and 3.0. ‒ Some pts need more anticoagulation and may have a goal PT/INR between 3.0 and 4.5. ‒ See Lehne p. 605 ‒ Longest reasonable amount of time between PT/INR labs is 2 – 4 weeks ‒ Patients daily dose of warfarin is titrated up or down based on PT/INR values • Antidote Vitamin K1 (phytonadione) • oral preferred (IV has risk of allergy or anaphylaxis) • Useful in emergencies (eg sudden trauma) • Therapeutic use: long-term prophylaxis of thrombosis • Prevention of venous thrombosis & associated risk of pulmonary embolism • Prevention of thromboembolism – Eg in patients with prosthetic heart valves • Prevention of thrombosis in patients with atrial fibrillation • Reduce recurrent Transient Ischemic Attacks and MI Warfarin, the first oral anticoagulant • Adverse effects – Bleeding – Hemorrhage – Fetal hemorrhage and teratogenesis • Pregnancy Category X • Contraindicated during lactation – Other adverse effects • Patient Teaching – S/S of bleeding; monitoring plan- schedule of labs; drug and food interactions; must tell all providers of all drugs; wear “MedicAlert”-type identification; stop warfarin (use another method) until after an elective surgery – Take at same time every day – Warfarin has perhaps the most drug or food interactions of any medication! Warfarin, the first oral anticoagulant • Drug interactions (p 606- 607) – Warfarin has perhaps the most drug and food interactions of any medication! – Worrisome due to increased bleeding or loss of protection – Drugs that increase anticoagulant effects • Heparin, aspirin, acetaminophen – Drugs that promote bleeding • Drugs that compete for binding with plasma proteins • Drugs that induce or inhibit the drug-metabolizing enzymes (CYP2C9) – Drugs that decrease anticoagulant effects • Contraindicated: very large list (p 607) • severe thrombocytopenia and others at high risk of bleeding • Patients undergoing a variety of procedures or surgeries • Liver disease, alcoholism- conditions that can disrupt production of clotting factors • Pregnancy and lactation 40 Warfarin, the first oral anticoagulant • Teach pt to ingest the same amount of dietary Vitamin K each day • Dietary vitamin K: Mayonnaise, canola oil, soybean oil, and green leafy vegetables 41 Question: warfarin and drug interactions Which patient does the nurse identify as most likely needing an increased dose of warfarin [Coumadin] to have the same anticoagulant effect? A.Patient taking acetaminophen [Tylenol] for back pain B.Patient taking cimetidine [Tagamet] to prevent gastric ulcers C.Patient taking oral contraceptives to prevent pregnancy D.Patient taking prednisone [Deltasone] for rheumatoid arthritis 42 HEPARIN VS. WARFARIN Comparison between Heparin & Warfarin MOA Route Onset Heparin Warfarin Inactivates thrombin & factor Xa injection SQ, IV Effects begin rapidly Inhibits synthesis of clotting factors oral Begin slowly Comparison between Heparin & Warfarin Heparin Warfarin Duration Monitoring Antidote Effects fade quickly Effects persist for days aPTT PT Protamine Sulfate Vitamin K Heparin and warfarin also have different therapeutic applications: •Heparin has immediate onset and is therefore used when anticoagulation needs to begin immediately (eg new diagnosis, or medical emergency). When used long-term, heparin has adverse effects (eg osteoporosis). Therefore is limited to short-term use. •Warfarin takes 2-5 days to reach therapeutic levels; therefore cannot be used for emergencies. However, warfarin is used when long-term Direct Thrombin Inhibitors • Dabigatran etexilate (Pradaxa) • Oral prodrug that undergoes conversion to dabigatran • Advantages: • Doesn’t require monitoring of anticoagulation • little risk of adverse interactions • same dose can be used for all patients regardless of age or weight • Therapeutic uses • Atrial fibrillation • Knee or hip replacement • Adverse effects • Bleeding • No specific antidote to reverse dabigatran-related bleeding • Gastrointestinal (GI) disturbances • Clinical considerations • Plasma levels peak about 1 – 3 hours after oral dosing. Not metabolized by liver, excreted by kidneys. Half-life 13 hours. Take dose at same time every day. No antidote Direct Factor Xa Inhibitors • Rivaroxaban [Xarelto] – Binds directly with factor Xa to cause inactivation – Prevention of DVT and PE after total hip or knee replacement surgery – Prevention of stroke in patients with atrial fibrillation – Treatment of DVT and PE unrelated to orthopedic surgery – Oral – No lab test – No antidote – Dosing at same time every day is required 47 Antiplatelet Drugs • Aspirin (ASA) – Inhibition of cyclooxygenase – Adverse effect • Increases risk for GI bleeding • Ticlopidine [Ticlid] – Inhibits ADP-mediated aggregation – Adverse effects • Hematologic effects • Clopidogrel [Plavix] – ADP receptor antagonist 49 Mechanism of platelet activation (causes stickiness and platelet aggregation.) Mechanisms of antiplatelet drugs are also depicted. Figure 52-1A ASPIRIN Antiplatelet Drugs: ASPIRIN • Aspirin (ASA) – Irreversible Inhibition of cyclooxygenase • Effects last ___ days? • Indications for use – Transient ischemic attack (TIA) – Primary prevention of Ischemic Stroke • Secondary prevention of Ischemic stroke ‒ Primary prevention of MI ‒ Secondary prevention of MI – Acute MI – Chronic stable angina – Unstable angina – Coronary stenting •Adverse effect – Bleeding; GI bleeding; Hemorrhagic stroke – Enteric-coated tablets may not reduce the risk of GI bleeding CLOPIDOGREL Antiplatelet Drugs: Clopidogrel • Clopidogrel [Plavix] – ADP receptor antagonist; Blocks P2Y12 ADP receptors on the platelet surface, preventing ADP-stimulated aggregation • Adverse effects: similar to aspirin • Bleeding • TTP (thrombotic thrombocytopenia purpura) • Drug Interactions: PPI’s, CYP2C19 inhibitors • Therapeutic uses – Prevents blockage of coronary artery stents – Reduces thrombotic events in patients with acute coronary syndromes • Prevents stenosis of coronary stents ‒ Also for secondary prevention of MI, ischemic stroke, and other vascular events • Use with caution in combination with other drugs that promote bleeding Antiplatelet Drugs: glycoprotein IIb/IIIa receptor antagonists • Glycoprotein (GP) IIb/IIIa receptor antagonists – Most effective antiplatelet drugs – “Super aspirins” – Reversible blockade of platelet GP IIb/IIIa receptors – Therapeutic use • Acute coronary syndromes • Percutaneous coronary interventions Thrombolytic Drugs • Alteplase [tPA] – Binds plasminogen Breaks down fibrin strands in the blood clot • Uses – Ischemic stroke- emergency management; must be administered within 2 hours of the onset of symptoms, preferably ASAP • Risk of bleeding increases as time passes after clot occurred – Massive pulmonary emboli – Acute coronary thrombosis (acute MI) – Deep venous thrombosis (DVT) – Clearing clogged central catheters • Adverse effects – Bleeding: Risk for intracranial bleeding higher than with streptokinase – If bleeding begins, treat with whole blood or blood products (packed red blood cells, fresh-frozen plasma) – Aminocaproic acid [Amicar] is last resort –Fever • Advantages – Does not cause allergic reactions – Does not induce hypotension Question During administration of alteplase [Activase], the patient’s IV site starts to ooze blood around the catheter. Which action by the nurse is most appropriate? A. Discontinue the infusion of alteplase. B. Assess the patient’s vital signs. C. Apply direct pressure over the puncture site. D. Administer aminocaproic acid [Amicar]. 58