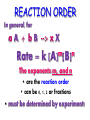* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download mechanisms - Manasquan Public Schools
Asymmetric induction wikipedia , lookup
Electrochemistry wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Process chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Stille reaction wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Click chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Rate equation wikipedia , lookup
George S. Hammond wikipedia , lookup
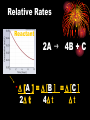
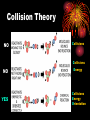
Chemical Kinetics Chapter 15 H2O2 decomposition in an insect H2O2 decomposition catalyzed by MnO2 Chemical Kinetics 2 • We can use thermodynamics to tell if a reaction is product or reactant favored. • But this gives us no info on HOW FAST reaction goes from reactants to products. •KINETICS — the study of REACTION RATES and their relation to the way the reaction proceeds, i.e., its MECHANISM. Energy Diagram KINETICS thermodynamics KINETICS the study of REACTION RATES and their relation to the way the reaction proceeds, i.e., its MECHANISM. •The reaction mechanism is our goal! 4 Reaction Mechanisms The sequence of events at the molecular level that control the speed and outcome of a reaction. Br from biomass burning destroys stratospheric ozone. (See R.J. Cicerone, Science, volume 263, page 1243, 1994.) Step 1: Br + O3 ---> BrO + O2 Step 2: Cl + O3 ---> ClO + O2 Step 3: BrO + ClO + light ---> Br + Cl + O2 NET: 2 O3 ---> 3 O2 5 REACTION RATES - RR = D [P ] = D [R ] Dt P =products Dt R = reactants Determining a Reaction Rate 7 Dye Concentration Rate = the change in [dye] divided by time The rate is determined from the plot. Time Relative Rates Reactant 2A g - 4B + C D [A ] = D [B ] = D [C ] 2D t 4D t Dt Rate Calculations Factors Affecting RXN Rates • *Nature of Reactants • Temperature • Concentration • Surface Area/ Physical state • Catalysts Collision Theory NO Collisions Collisions NO YES Energy Collisions Energy Orientation 12 Concentrations and Rates To postulate a reaction mechanism, we study • reaction rate and • its concentration dependence 13 Concentration and rate What is concentration of reactant as function of time? The rate law is D[A] Rate = k [A] Dtime REACTION ORDER In general, for a A + b B --> x X Rate = k m n [A] [B] The exponents m,, and n • are the reaction order • can be 0, 1, 2 or fractions • must be determined by experiment! Rate contant: Arrhenius equation Rate constant is dependent on only the activation energy and temperature Temp (K) Rate constant k Ae Frequency factor -E a / RT Activation energy 8.31 x 10-3 kJ/K•mol Frequency factor = frequency of collisions with correct geometry. Simulation: RATE MECHANISMS A Microscopic View of Reactions Mechanism: how reactants are converted to products at the molecular level. RATE LAW ----> MECHANISM experiment ----> theory 17 More on Mechanisms Reaction is UNIMOLECULAR if only one reactant is involved. BIMOLECULAR if two different molecules must collide to form a products 18 A bimolecular reaction Collision Theory Reactions require (a) activation energy and (b) correct geometry. O3(g) + NO(g) ---> O2(g) + NO2(g) 1. Activation energy 2. Activation energy and geometry 19 Mechanisms O3 + NO reaction occurs in a single ELEMENTARY step. Most others involve a sequence of elementary steps. Adding elementary steps gives NET reaction. 20 21 2 I- + 2 H+ + H2O2---> I2 + 2 H2O 1. Rate law determined from experiment is: Rate = k [I-] [H2O2] Most rxns. have sequence of elementary steps. NOTE Order and stoichiometric coefficients NOT the same! 3. Rate law reflects all chemistry down to and including the slowest step in multistep reaction. 2. Mechanisms 22 2 I- + H2O2 + 2 H+ ---> I2 + 2 H2O Rate = k [I-] [H2O2] Proposed Mechanism Step 1 — slow HOOH + I- --> HOI + OH- Step 2 — fast HOI + I- --> Step 3 — fast 2 OH- + 2 H+ I2 + OH--> 2 H2O Rate is controlled by slow step — RATE DETERMINING STEP, RDS. Rate can be no faster than RDS! Mechanisms 23 2 I- + H2O2 + 2 H+ ---> I2 + 2 H2O Rate = k [I-] [H2O2] Step 1 — slow Step 2 — fast Step 3 — fast HOOH + I- --> HOI + OH- HOI + I- --> I2 + OH2 OH- + 2 H+ --> 2 H2O Elementary Step 1 is bimolecular and involves Iand HOOH. Therefore, this predicts the rate law should be Rate [I-] [H2O2] — as observed!! The species HOI and OH- are reaction intermediates. 24 Simulation:” Mechanisms 25 Sovled problems: pg 144 NO2 + CO NO + CO2 26 Single step NO2 + CO reaction: Rate = k[NO2]2 Two possible mechanisms Two steps: step 1 Two steps: step 2 27 Ozone Decomposition Mechanism 2 O3 (g) ---> 3 O2 (g) [O3 ]2 Rate = k [O2 ] Proposed mechanism Step 1: fast, equilibrium O3 (g) <--> O2 (g) + O (g) Step 2: slow O3 (g) + O (g) ---> 2 O2 (g) 28