* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Oxidation-Reduction (Redox) Reactions
Hydrogen-bond catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Water splitting wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Periodic table wikipedia , lookup
Double layer forces wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Theory of solar cells wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Marcus theory wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Coordination complex wikipedia , lookup
Geochemistry wikipedia , lookup
Electron configuration wikipedia , lookup
Chemical reaction wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metallic bonding wikipedia , lookup
Atomic theory wikipedia , lookup
Gaseous detection device wikipedia , lookup
History of electrochemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Oxidation state wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
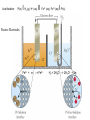
Oxidation-Reduction (Redox) Reactions and Electrochemistry Dr. Joshi Graphics and examples taken from Chemistry, 3rd ed by Olmsted and Williams. Electrochemistry • Study of interchange between chemical and electrical energy • Use or generation of electric current in conjunction with chemical reaction What is redox? • • • • • • • • • • • • Oxidation is the loss of electron(s). Reduction is the gain of electron(s). LEO says GER The two processes occur together. 2 Mg (s) + O2 (g) --> 2 MgO (s) Mg --> Mg2+ + 2 e(half-reaction) (half-reaction) O2 + 4 e- --> 2 O2How do these half reactions get put together? Which species is oxidized? Which is reduced? Which is the oxidizing agent? Which is the reducing agent? One of these is a redox reaction, and the other is not. How can you tell which is which? You need to determine which species, if any, has lost electrons and which, if any, has gained electrons. We do this by assigning oxidation numbers. Rules for assigning oxidation states (oxidation numbers): 1. An atom in its elemental state has an oxid. state of zero. e.g. Na, H2, O2, Br2, Mg 2. An atom in a monatomic ion has oxid. number identical to its charge. e.g. Na+ is +1, Al3+ is +3, Cl– is –1 You already know what types of ions many of the elements tend to form! 3. An atom in a polyatomic ion or a compound has the same oxid. # it would have if it were an ion. e.g. MgCl2 Mg is +2, and Cl is –1. 4. Oxygen is almost always –2. O takes precedence over halogens and H. (Exception: peroxide, an O-O bond. You won’t run into this very much.) 5. Halogens are –1. Exception: when bound to O. O takes precedence! Cl–O–Cl Each Cl is +1 b/c O has to be –2. 6. H is typically +1 when it’s with nonmetals and –1 when it’s with metals. Oxygen and metals take precedence. H2O O has to be –2, so each H is +1. NaH Na has to be +1 (alkali metal), so H is –1. 7. Left side of periodic table (metals) cation-like and right side (nonmetals) anion-like. 8. Sum of oxidation numbers equals overall charge. Advice: Remember which elements take precedence, and make the others adjust. In a compound or as an ion, alkali metals will always be +1. In a compound or an ion, alkaline metals will always be +2. (The other atoms in the compound have to adjust their oxidation states to give the correct overall charge. e.g. NaH) O is –2 (unless two Os bonded to each other) (Everything else in the compound must adjust.) Assign oxidation numbers (states) to determine which is the redox reaction. Which species is oxidized? Which is reduced? Balancing Redox Reactions Step 1: Break the unbalanced equation into half-reactions. Step 2: Balance each half-reaction by following a-d. a. Balance all elements except O and H by adjusting coefficients. b. Balance O by adding H2O to the appropriate side. c. Balance H by adding the appropriate number of H+ ions to the side that needs H. Add H2O to the other side if needed. d. Balance net charge by adding electrons. Step 3: Multiply the half-reactions by integers that will allow for cancellation of electrons. Step 4: Combine half-reactions, and simplify by combining and canceling duplicated species. Step 5 (Only if the solution is under basic conditions): Add enough OH- (to both sides) to neutralize any H+ ions. Simplify by canceling duplicate species (if needed). Balance the following redox reaction (first under acidic/neutral conditons, then under basic conditions). Cu (s) + NO3— (aq) --> Cu2+ (aq) + NO (g) Which metal is oxidized? Which is reduced? Is this process spontaneous? Oxidation occurs at the anode. Reduction occurs at the cathode. The anode and cathode are electrodes. Schematic view of an arrangement that allows a sustained redox reaction accompanied by an external flow of electrons. Using Standard Reduction Potentials Zn2+(aq) + 2 e- (wire) Zn(s) Cu2+(aq) + 2 e- (wire) Eo = + 0.76 V Cu(s) Eo = + 0.34 V See table of Standard reduction potentials. Change sign of E when reverse the half-reaction. E does NOT change when half-rxn is multiplied! Add up E values when add half rxns together. DGo = - nFE o n = # of mol of eF = Faraday’s constant = 96,485 C/mol ePositive cell potential gives negative DG. So, positive E means the process is spontaneous. Standard conditions: 1 M, 1 atm Line Notation: Pt(s) IH 2 (g), H+ (aq) II Fe 3+ (aq), Fe2+ (aq) I Pt(s) Passive Electrodes Fe3+ + e- --> Fe2+ H2 + 2H2O --> 2H3O+ + 2e- A galvanic cell runs spontaneously (E is positive). An electrolytic cell does not run spontaneously (E is negative). To run an electrolytic cell, electric current must be supplied. Nernst Equation: Cell Potentials Under Non-Standard Conditions E = Eo – RT ln (Q) nF For a cell at equilibrium, E=0 and Q=K log (K) = nEo 0.0591