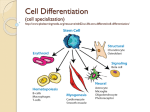
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Molecular mechanisms of sex determination and gonadal sex
Genome (book) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene expression programming wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Fish Physiol Biochem (2005) 31:105–109 DOI 10.1007/s10695-006-7590-2 RESEARCH ARTICLE Molecular mechanisms of sex determination and gonadal sex differentiation in fish Y. Nagahama Ó Springer Science+Business Media B.V. 2006 Abstract We have used various genetic and molecular approaches to investigate the mechanisms of sex determination and gonadal sex differentiation in fish. DMY was identified as the sex-determining gene of medaka. In tilapia, endogenous estrogens act as natural inducers of ovarian differentiation, while DMRT1 may be important for testicular differentiation. The roles of these regulators in sex determination and gonadal sex differentiation were ascertained using a gene or hormonal blockade strategy. Keywords Fish Æ Sex-determining gene Æ Estrogen Æ DMRT1 Æ Gonadal sex differentiation Introduction In vertebrates, sex determination and differentiation are key events in the development of either the testis or ovary. SRY/Sry (sex determining region Y chromosome) is the Y chromosome gene responsible for Y. Nagahama (&) Laboratory of Reproductive Biology, Department of Developmental Biology, National Institute for Basic Biology, Okazaki 444-8585, Japan Y. Nagahama CREST, Kawaguchi 332-0012, Japan e-mail: [email protected] initiating testicular development in mammals (Sinclair et al. 1990; Koopman et al. 1991). The collection of molecular candidates implicated in the process of gonadal sex differentiation is now quite extensive. However, one is not able to fit all of these into simple testicular and ovarian pathways (Koopman 2001; Nakagawa 2004; Smith and Sinclair 2004; Yao 2005). In fish, sexual characteristics and gonadal development vary from gonochoristic species to several types of hermaphroditism (Devlin and Nagahama 2002). Thus, a knowledge of sex determination and differentiation in fish will broaden our understanding of these processes beyond the specific details found within the group. We have been investigating the molecular mechanisms of sex determination and gonadal sex differentiation using two fish species, the medaka, Oryzias latipes (sex determination) and tilapia Oreochromis niloticus (gonadal sex differentiation). We are particularly interested in identifying genes or hormonal factors that may regulate the early development of fish gonads. Sex determination In addition to its small size and short generation time, the medaka has two major advantages for genetic research: a large interstrain diversity within the species and the existence of several inbred strains (Schartl 2004; Matsuda 2005). As in mammals, sex 123 106 determination in medaka is male heterogametic (a stable genetic XX/XY sex determining system). Based on positional cloning and a detailed analysis of BAC clones (Matsuda et al. 2001) using shotgun sequencing, we located a unique gene in the short sex-determining region on the Y chromosome. This gene consists of six exons and encodes a protein of 267 amino acids including the highly conserved DM domain. The DM domain was named after a related DNA-binding motif found in two proteins, doublesex (dsx) and mab-3, involved in sexual development in Drosophila and C. elegans, respectively. This Yspecific DM-domain gene was named DMY (DMdomain gene on the Y chromosome) (Matsuda et al. 2002; Matsuda 2005), or Dmrt1b (Nanda et al. 2002; Volff et al. 2003). Two naturally occurring XY female medaka showed different mutations in the DMY gene (Matsuda et al. 2002). One of these mutants was found to carry a mutation causing a frameshift and premature termination of the DMY protein. When mated, all XY offspring with the mutant Y were female (Matsuda et al. 2002). The other mutant had a severe depression in DMY expression in the embryo and 60% of its XY offspring with the mutant Y developed as females (Matsuda et al. 2002). Taken together, the loss-offunction mutant and the depressed expression mutant strongly indicate that DMY is required for normal testicular development. More recently, we demonstrated that a genomic DNA fragment carrying DMY, containing about 56 kb of the coding region, about 60 kb of the upstream non-coding region, and about 1.4 kb of the downstream non-coding region (Matsuda et al., unpublished). Among these transgenic progeny, we found that 25% of XX fish develop into males. Furthermore, some males were found to be fertile. These findings demonstrate that DMY is sufficient to induce male development in XX medaka. It is important to note that DMY transgenic XX medaka are fully functional and fertile males, whereas Sry transgenic mice are sterile (Koopman et al. 1991). Thus, medaka is the first transgenic vertebrate shown to undergo a complete sex reversal. Taken together, these loss- and gain-of-function studies indicate that DMY is the sex-determining gene of medaka. DMY is thus the first sex-determining gene to be found in non-mammalian vertebrates (Swan 2002; Schartl 2004; Matsuda 2005). 123 Fish Physiol Biochem (2005) 31:105–109 Y chromosome-linked DMY appears to have originated from a duplicate copy of autosomal DMRT1 (DM-related transcription factor 1), another DM domain gene that is most homologous to DMY (about 80% at the amino-acid level) and is involved in male development in various vertebrates (Nanda et al. 2002; Lutfalla et al. 2003). Then, the duplicated DMRT1 in the Y chromosome acquired a new function as a sex-determining gene, DMY (Matsuda 2005). Further, DMY is also found in Oryzias curvinotus, which is most closely related to medaka (Matsuda et al. 2003), but is not found in other Oryzias species or in other fishes (Kondo et al. 2003) (Table 1). Importantly, there is no sequence homology between two known sex-determining genes, SRY/Sry and DMY (Sinclair et al. 1990; Matsuda et al. 2002; Nanda et al. 2002). Taken together, these findings suggest that the top of the cascades leading to males and females, the sex-determining gene, exhibits extensive diversity among vertebrates. In medaka, the first apparent morphological sex difference is in the number of germ cells immediately before hatching (Kobayashi et al. 2004). In XY fry, primordial germ cells (PGCs) subsequently enter mitotic arrest and the proliferation of A-type spermatogonia does not occur until 20–30 days after hatching (dah). Because of the sequence similarity between DMY and DMRT1, expression of DMY and DMRT1 cannot be distinguished by in situ hybridization, but can be distinguished by RT-PCR using specific primers. DMY mRNA and protein are expressed specifically in the somatic cells (Sertoli cells) surrounding PGCs in the early gonadal primordium, before morphological sex differences are apparent Table 1 SRY/Sry, DMY and DMRT1 in various vertebrate species Fishes Oryzias latipes Oryzias curvinotus Oryzias celebensis Fugu Zebrafish Tilapia Amphibians Reptiles Birds Mammals SRY/Sry DMY DMRT1 ) ) ) ) ) ) ) ) ) + + + ) ) ) ) ) ) ) ) + + + + + + + + + + Fish Physiol Biochem (2005) 31:105–109 (immediately before hatching). DMY protein is localized to the nuclei of Sertoli cells, suggesting that DMY functions as a transcriptional regulator during early testicular differentiation. RT-PCR analyses further show that expression of DMY in the testis is continuous from the embryonic to adult stage. No specific expression of DMRT1 is observed in either sex during gonadal sex differentiation. DMRT1 is expressed in spermatogonium-supporting cells after testicular differentiation (20–30 dah) and its level is much higher than that of DMY in mature testes. In XX sex-reserved testes, DMRT1 is expressed in the Sertoli cell lineage, similar to the expression of DMY in XY testes. Taken together, these results strongly suggest that in medaka, DMY regulates PGC proliferation and testicular differentiation, and that DMRT1 is a regulator of spermatogenesis. Gonadal sex differentiation Unlike in mammals, in non-mammalian vertebrates sex steroids play important roles in gonadal sex differentiation. The roles of sex steroids in sex determination and differentiation in fish have been comprehensively reviewed (Yamamoto 1969; Nakamura et al. 1998; Baroiller et al. 1999; Guiguen 2000; Piferrer 2001; Devlin and Nagahama 2002; Strussmann and Nakamura 2003). The Nile tilapia provies an excellent example of the precise nature of the steroidogenic actions during gonadal sex differentiation (Nakamura et al. 1998; Nagahama 2000; Kobayashi et al. 2003). In this fish, all genetic female (XX) or male (XY) broods can be obtained from sexreversed, pseudo male sperm (XX), or normal eggs (XX) and super male sperm (YY), respectively. The gonadal anlage is not observed in tilapia fry at hatching and primordial germ cells (PGCs) are located in the outer layer of the lateral plate mesoderm. At 3 dah, the gonadal anlagen forms and the germ cells in it are enclosed by somatic cells derived from the coelomic epithelium (Kobayashi et al. 2000). Using genetically controlled all-female and all-male tilapia, we showed that cells immunopositive for four steroidogenic enzymes essential for the biosynthesis of all major sex steroid hormones including androgens and estrogens, appeared first in the female gonadal anlargen of tilapia at 7–10 dah. Immunopositive 107 steroid-producing cells increased in number accompanying ovarian differentiation and increased more in the developed ovaries (Nakamura et al. 1998; Kobayashi et al. 2003). Real-time PCR analysis further revealed that oP450arom (the ovarian form of aromatase) mRNA was first detected at 5 dah in female gonads, and then increased rapidly at 7 dah (S. Ijiri and H. Kaneko, unpublished). We also found that estrogen receptors a and b are expressed before the expression of steroidogenic enzymes in XX fry (T. Kobayashi et al., unpublished). These findings strongly suggest that estrogen (estradiol-17b) is produced in the gonads around the time of ovarian differentiation and may have an important role in the differentiation. In tilapia, treatment of genetic females with a non-steroidal P450arom inhibitor (fadrozole) before and during gonadal sex differentiation causes female masculinization (sex reversal from females to males), suggesting that endogenous estrogens are already synthesized in undifferentiated gonads of genetic females (Nakamura et al. 1998). In contrast, all fish treated with both fadrozole and estradiol-17b had normal ovaries. In addition, treatment of genetic females with an estrogen receptor antagonist (tamoxifen) results in their masculinization. These findings further strengthen the idea that endogenous estrogen signaling through estrogen receptors plays key roles in ovarian differentiation in tilapia (Fig. 1). Medaka Y X DMY DMY X Tilapia X ? Foxl2 F oxl2 oP450arom oP450arom X Y X X ? ? DMR T1 DMRT1 F oxl2 Foxl2 oP450arom Testis Ovary DMR T1 DMRT1 Testis Ovary Fig. 1 A potential mechanism of sex determination and gonadal sex differentiation in medaka and tilapia 123 108 In contrast to XX fry, immunopositive reactions to antibodies against several steroidogenic enzymes were not observed in undifferentiated gonads of genetic males. Weakly positive cells appeared first after testicular differentiation and strong reactions, except for oP450arom, were seen in the testes just before the onset of spermatogenesis. Thus, it is less likely that steroid hormones, including androgens, have an important role in testicular differentiation in tilapia. A good candidate for a factor regulating testicular differentiation is DMRT1 (Guan et al. 2000; Devlin and Nagahama 2002). We found that the DMRT1 transcript was expressed male-specifically in testicular Sertoli cells of tilapia prior to and during sex differentiation. More recently, we have shown that genetically female tilapia carrying extra copies of tilapia DMRT1 as a transgene undergo sex reversal (D.S. Wang et al., unpublished). These results strongly suggest an important role for DMRT1 in testicular differentiation in tilapia (Fig. 1). Since endogenous estrogens are critical for directing initial ovarian differentiation, the genes regulating oP450arom expression are keys to gonadal sex differentiation in fish. However, the regulatory factor activating oP450arom during ovarian differentiation is at present unknown. We have previously shown that Ad4BP/SF-1 acts as an important transcriptional modulator of gonadotropin-induced oP450arom gene expression in vitellogenic follicles of tilapia (Yoshiura et al. 2003). Although the Ad4BP/SF-1 transcript is detected by RT-PCR, no difference in expression is observed between males and females during sex determination and differentiation. More recently, we found that the forkhead transcription factor Foxl2 and oP450arom begin to be expressed at the same time (5 dah) in genetic females and their levels increased at 7 dah. Luciferase assays using mammalian cell lines showed that both Ad4BP/ SF-1 and Foxl2 activate transcription of the oP450arom gene. Promoter assays and electromobility shift assays revealed that Ad4BP/SF-1 and Foxl2 most likely act by binding directly to the oP450arom promoter. We also found that Foxl2 enhances Ad4BP/SF-1-activated transcription of oP450arom. Our pull-down assays suggest that Foxl2 can heterodimerize with Ad4BP/SF-1. In vivo transgenic over-expression of a dominant negative mutant of Foxl2 was achieved by injection of these GFP constructs into fertilized XX eggs. These transgenic 123 Fish Physiol Biochem (2005) 31:105–109 XX tilapia showed a reduced expression of oP450arom, leading to ovarian degeneration, followed by the development of testicular tissue to different degrees and, in some cases, a complete sex change (D.S. Wang et al., unpublished). Our recent findings also suggest that Dmrt1 may be involved in the regulation of oP450arom gene expression (D.S. Wang et al., unpublished). Luciferase assays revealed that Dmrt1 represses Ad4BP/ SF-1-activated transcription of oP450arom. Our data suggest that DMRT1 does not bind the oP450arom promoter, but can heterodimerize with Ad4BP/SF-1. Over-expression of Dmrt1 in XX gonads resulted in reduced expression of oP450arom with gonadal structures similar to those seen in transgenic XX gonads with a dominant negative mutant of Foxl2. Taken together, these in vivo and in vitro results strongly suggest that the transcriptional regulation of oP450arom by Foxl2 (stimulatory) (Fig. 1) and Dmrt1 (inhibitory) is critical for gonadal sex differentiation in the Nile tilapia. Conclusions In this brief review, three major factors, DMY, DMRT1 and estrogens (oP450arom), were discussed in relation to sex determination, testicular differentiation and ovarian differentiation, respectively. Our findings, together with earlier studies on other vertebrates, suggest that the top of the cascades leading to males and females, the sex-determining gene, exhibits extensive diversity among vertebrates (Table 1), while the molecular cascades leading to males and females seem to be relatively conserved (Fig. 1). Preliminary data from our laboratory also suggest that several transcription factors such as DMY, Ad4BP/SF-1, Dax1 and Dax2 may be involved in gonadal sex differentiation in both medaka and tilapia (D.S. Wang and L.Y. Zhou, unpublished). Studies on a coordinated interaction between the transcription and hormonal factors may be important to understand the mechanisms that lead to testicular and ovarian differentiation. Among the vertebrates, fish exhibiting a range of gonadal differentiation types including gonochoristic species as well as hermaphroditic species will continue to provide an excellent model for the study of sex determination and gonadal sex differentiation. Fish Physiol Biochem (2005) 31:105–109 Acknowledgements Work from this laboratory presented in this review was supported, in part, by the CREST and SORST Research Projects of Japan Science and Technology Corporation, and Environmental Endocrine Disruptor Studies from the Ministry of the Environment. References Baroiller JF, Guiguen Y, Fostier A (1999) Endocrine and environmental aspects of sex differentiation in fish. Cell Mol Life Sci 55:910–931 Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish. Aquaculture 208:191–366 Guan G, Kobayashi T, Nagahama Y (2000) Sexually dimorphic expression of DM (Doublesex/Mab-3)-domain genes during gonadal differentiation in tilapia. Biochem Biophys Res Comm 272:662–666 Guiguen Y (2000) Implication of steroids in fish gonadal sex differentiation and sex inversion. Curr Topic Steroid Res 3:127–143 Kobayashi T, Kajiura-Kobayashi H, Nagahama Y (2000) Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis of a teleost fish, tilapia, Oreochromis niloticus. Mech Develop 99:139–142 Kobayashi T, Kajiura-Kobayashi H, Nagahama Y (2003) Induction of XY sex reversal by estrogen involves altered gene expression in a teleost, tilapia. Cytogenet Genome Res 101:289–294 Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y (2004) Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dyn 231:518–526 Kondo M, Nanda I, Hornung U, Asakawa S, Shimizu N, Mitani H et al (2003) Absence of the candidate male sex-determining gene dmrt1b(Y) of medaka from other fish species. Curr Biol 13:416–420 Koopman P (2001) The genetics and biology of vertebrate sex determination. Cell 105:843–847 Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromsomally female mice transgenic for Sry. Nature 351:117–121 Lutfalla G, Roest Crollius H, Brunet FG, Laudet V, RobinsonRechavi M (2003) Inventing a sex-specific gene: a conserved role of DMRT1 in teleost fishes plus a recent duplication in the medaka Oryzias latipes resulted in DMY. J Mol Evol 57(Suppl. 1):S148–S153 Matsuda M (2005) Sex determination in the teleost medaka, Oryzias latipes. Ann Rev Genet 39:293–307 Matsuda M, Kawato N, Asakawa S, Shimizu N, Nagahama Y, Hamaguchi S, Sakaizumi M, Hori H (2001) Construction of a BAC library derived from the inbred Hd-rR strain of the teleost fish, Oryzias latipes. Gene Genet Syst 76:61–63 109 Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M (2002) DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417:559–563 Matsuda M, Sato T, Toyazaki Y, Nagahama Y, Hamaguchi S, Sakaizumi M (2003) Oryzias curvinotus has DMY, a gene that is required for male development in the medaka, O. latipes. Zool Sci 20:159–161 Nagahama Y (2000) Gonadal steroid hormones: major regulators of gonadal sex differentiation and gametogenesis in fish. In: Norberg B, Kjesbu OS, Taranger GL, Anderson E, Stefansson SO (eds) Proc. Sixth Int. Symp. Reprod. Physiol. Fish. Univ. Bergen. pp 211–222 Nakagawa S (2004) Is avian sex determination unique?: clues from a warbler from chickens. Trends Genet 20:479–480 Nakamura M, Kobayashi T, Chang XT, Nagahama Y (1998) Gonadal sex differentiation in teleost fish. J Exp Zool 281:362–372 Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M (2002) A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA 99:11778– 11783 Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281 Schartl M (2004) A comparative view on sex determination in medaka. Mech Dev 121:639–645 Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf A-M, Lovell-Badge R, Goodfellow PN (1990) A gene from the human sex determining region encodes a protein with homology to a conserved DNA binding motif. Nature 346:240–244 Smith CA, Sinclair AH (2004) Sex determination: insights from the chicken. BioEssays 26:120–132 Strussmann CA, Nakamura M (2003) Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol Biochem 26:13–29 Swan A (2002) Vertebrate sex determination: a new player in the field. Curr Biol 12:R602–R603 Volff JN, Kondo M, Schartl M (2003) Medaka dmY/dmrt1Y is not the universal primary sex-determining gene in fish. Trends Genet 19:196–199 Yamamoto T (1969) Sex differentiation. In: Hoar WS, Randall DJ (eds) Fish physiology, vol III. Academic Press, pp 117–175 Yao HHC (2005) The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol 230:87–93 Yoshiura Y, Senthilkumaran B, Watanabe M, Oba Y, Kobayashi T, Nagahama Y (2003) Synergistic expression of Ad4BP/SF-1 and cytochrome P-450 aromatase (ovarian type) in the ovary of Nile tilapia, Oreochromis niloticus, during vitellogenesis suggests transcriptional interaction. Biol Reprod 68:1545–1553 123