* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Formation of C-C Bonds via Catalytic Hydrogenation and Transfer
Strychnine total synthesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Kinetic resolution wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
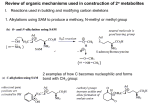
Formation of C-C Bonds via Catalytic Hydrogenation and Transfer Hydrogenation Professor Michael J. Krische, Ph.D. Department of Chemistry, University of Texas at Austin [email protected] Abstract: Carbon-carbon bond formation lies at the heart of chemical synthesis. Research in the Krische laboratory reveals reductive C-C bond formation can be achieved under the conditions of catalytic hydrogenation. This concept is extended further via “C-C bond forming transfer hydrogenations”, wherein hydrogen exchange between alcohols and π-unsaturated reactants triggers generation of aldehyde-organometal pairs that combine to give products of carbonyl addition. Direct alcohol CHfunctionalization via redox-triggered carbonyl addition enhances efficiency by merging alcohol-toaldehyde redox reactions with C-C bond construction events. This new pattern of reactivity enables the direct conversion of lower alcohols to furnish higher alcohols in the absence of premetallated reagents. These methods have been applied to the construction of diverse polyketide natural products. In each case, the most concise route to any member of the respective natural product family was achieved