* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
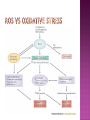
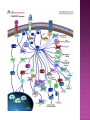
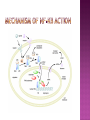
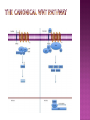
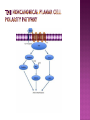
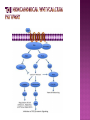
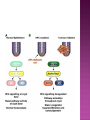
Ms. Bushra ALLAH RAKHA Ph.D. SCHOLAR DEPARTMENT OF WILDLIFE MANAGEMENT PMAS-AAUR ROS are generally perceived as toxicants that induce various deleterious effects, like cell dysfunction, death or malignant transformation. The toxic potential of ROS is used by the innate immune defense as a powerful weapon against pathogens. If pathogens may elude their recognition as “non-self” by the sophisticated adaptive immune system due to their plasticity and adaptive mechanisms, they cannot escape the rough chemical attack of ROS. Cellular redox balance is maintained by a powerful antioxidant system that “neutralizes” ROS. It consists of SOD, catalase, the glutathione system (glutathione, glutathione reductase, peroxidase and transferase), the thioredoxin system (thioredoxins, thioredoxin peroxidase and peroxiredoxins), vitamin E and C. Subtle intra- and extracellular mechanisms related to metal-binding proteins (transferring, albumin, ferritin etc.) and various metabolites (uric acid, bilirubin, pyruvate etc) are also active. It is noteworthy that cellular targets attacked and damaged by ROS (lipids, proteins, sugars, nucleic acids) contribute themselves to ROS detoxification and represent therefore sacrifice cellular components. Oxidative stress generally describes a condition in which cellular antioxidant defense mechanisms are insufficient to inactivate ROS, or excessive ROS are produced, or both. It is well-documented that significant oxidative stress carries out severe damage to lipids, proteins, sugars and nucleic acid bases which compromises cell viability and functions. Oxidative damage of any cellular constituent, if unchecked, can theoretically contribute to disease development. Indeed, an increasing amount of evidence suggests that oxidative stress is linked, more or less directly, to either primary or secondary pathophysiologic mechanisms of several acute and chronic human diseases. cancer cells develop an enhanced constitutive oxidative stress that sustains tumor growth and shields these cells against pro-apoptotic signals, thus promoting tumor progression. Rac1 is a small G-protein in the Rho family that drives Actin polymerization and formation of lamellipodia, promotes cell-cell adhesion, breakdown and migration of different carcinoma cells. In other epithelial cell lines, Rac activation also plays a role in scattering after distinct stimuli such as Growth factor stimulation or Integrin engagement. It also plays a critical role in processes, such as control of cell morphology, transcriptional activation, and apoptosis signaling. The broad range of events controlled by this GTPase requires regulation of its interactions with multiple downstream targets. Rac1 is activated by GEF (Guanine Nucleotide Exchange Factors), in particular ARHGEF6 and repressed by GAPs (GTPase-Activating Proteins), specifically RacGAP. Rac1 GTPase mediates key cellular processes in response to upstream regulators such as Growth Factors, Integrins and HA (Hyaluronic Acid)-binding receptor CD44. Signaling pathways that are regulated by Rho family members play an important role in several pathological conditions, including cancer, inflammation, and bacterial infections. Rac controls the generation of ROS , both in leukocytes and nonhematopoietic cells and is necessary for Cadherin-dependent adhesion. Rac activation is required for the full transformed phenotype induced by oncogenes such as TIAM1 and Ras. In addition, Rac activation perturbs cadherin contacts with a concomitant change in cell shape, including formation of lamellae/protusions and conversion to a fibroblastic morphology. Translocation of Rac to the plasma membrane is also required for assembly and activation of the NADPH Oxidase complex. The importance of Rac1 in neoplastic transformation is indicated by the finding that Rac1 is necessary for K-Ras-induced transformation of fibroblasts. Nuclear factor kappaB (NFκB) is also implicated in cancer development and progression. NFκB is a ubiquitously expressed transcription factor which regulates over 200 different genes involved in numerous pathways including inflammation, apoptosis/survival, cell cycle progression and migration. Interestingly, many cancers have been shown to rely on constitutive NFκB signaling, which is now referred to as NFκB addiction. Protein complex that controls the transcription of DNA. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, UV radiation, oxidized LDL, and bacterial or viral antigens. NF-κB plays a key role in regulating the immune response to infection (κ light chains are critical components of immunoglobulins). Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory. NF-κB (green) heterodimerizes with RelB (cyan) to form a ternary complex with DNA (orange) that promotes gene transcription. NF-κB is important in regulating cellular responses because it belongs to the category of "rapid-acting" primary transcription factors, i.e., transcription factors that are present in cells in an inactive state and do not require new protein synthesis in order to become activated (other members of this family include transcription factors such as c-Jun, STATs, and nuclear hormone receptors). This allows NF-κB to be a first responder to harmful cellular stimuli. Known inducers of NF-κB activity are highly variable and include reactive oxygen species (ROS), tumor necrosis factor alpha (TNFα), interleukin 1-beta (IL1β), bacterial lipopolysaccharides (LPS), isoproterenol, cocaine, and ionizing radiation. NF-κB is widely used by eukaryotic cells as a regulator of genes that control cell proliferation and cell survival. Active NF-κB turns on the expression of genes that keep the cell proliferating and protect the cell from conditions that would otherwise cause it to die via apoptosis. Defects in NF-κB results in increased susceptibility to apoptosis leading to increased cell death. This is because NF-κB regulates anti-apoptotic genes especially the TRAF1 and TRAF2 and, therefore, checks the activities of the caspase family of enzymes, which are central to most apoptotic processes. In tumor cells, NF-κB is active either due to mutations in genes encoding the NF-κB transcription factors themselves or in genes that control NF-κB activity (such as IκB genes). Some tumor cells secrete factors that cause NF-κB to become active. Blocking NF-κB can cause tumor cells to stop proliferating, to die, or to become more sensitive to the action of anti-tumor agents. The Wnt signaling pathways are a group of signal transduction pathways made of proteins that pass signals from outside of a cell through cell surface receptors to the inside of the cell. Three signaling pathways: The canonical Wnt pathway The noncanonical planar cell polarity pathway The noncanonical Wnt/calcium pathway. All three Wnt signaling pathways are activated by the binding of a Wnt-protein ligand to a Frizzled family receptor, which passes the biological signal to the protein Dishevelled inside the cell. The canonical Wnt pathway leads to regulation of gene transcription. The noncanonical planar cell polarity pathway regulates the cytoskeleton that is responsible for the shape of the cell The noncanonical Wnt/calcium pathway regulates calcium inside the cell. Wnt signaling was first identified for its role in carcinogenesis, but has since been recognized for its function in embryonic development. The clinical importance of this pathway has been demonstrated by mutations that lead to a variety of diseases, including breast and prostate cancer, colorectal cancer, glioblastoma, type II diabetes. Colorectal cancer, also known as colon cancer, rectal cancer, or bowel cancer, is a cancer from uncontrolled cell growth in the colon or rectum (parts of the large intestine), or in the appendix. The symptoms and signs of colorectal cancer depend on the location of tumor in the bowel, and whether it has spread elsewhere in the body (metastasis). The classic warning signs include: worsening constipation, blood in the stool, decrease in stool calibre, loss of appetite, loss of weight, and nausea or vomiting in someone over 50 years old. Greater than 75-95% of colon cancer occurs in people with little or no genetic risk. Other risk factors include older age, male gender, high intake of fat, alcohol or red meat, obesity, smoking and a lack of physical exercise. Approximately 10% of cases are linked to insufficient activity. Those with a family history in two or more firstdegree relatives have a two to threefold greater risk of disease and this group accounts for about 20% of all cases. A number of genetic syndromes are also associated with higher rates of colorectal cancer. Colorectal cancer (CRC) derives from normal colonic mucosa by stepwise accumulation of somatic genetic alterations, starting with inactivation of the tumor suppressor gene Adenomatous Polyposis Coli (APC) or activation of the oncogene ß-catenin (CTNNB1). APC is a negative regulator of WNT signaling and its mutational inactivation leads to accumulation of β-catenin, which associates with transcription factors of the T-cell Factor (TCF) family to activate WNT target genes. RAC1 acts downstream of constitutive WNT signaling to promote progenitor cell proliferation and expansion of the LGR5 ISC population. Mechanistically, these phenotypes depend on RAC1-driven production of ROS and NF-kB signaling. The role of ROS in stem cell maintenance, cellular transformation, and CSC survival appears to be context and tissue specific. In proliferative neural stem cells, high levels of ROS regulate self-renewal by driving PI3K/AKT signaling. Conversely, hematopoietic stem cells are sensitive to ROS levels and higher levels limit their lifespan. RAC1 is also required for efficient NF-kB signaling after Apc loss and, importantly, constitutive activation of NF-kB in the absence of RAC1 partially rescued the attenuated proliferation and ISC expansion phenotypes. RAC1 deficiency prevents this by impairing NF-kB signaling, despite b-catenin nuclear accumulation. The finding that ROS and NF-kB both play important roles in intestinal tumor initiation indicates a role for inflammation in this process. Rac1 deletion did not completely recapitulate this, the comprehensive suppression of tumor formation suggests that RAC1 is required for transformation after Apc loss. ROS and NF-kB are two parallel pathways involved in promoting ISC/progenitor proliferation downstream of RAC1. It should be noted that activation of ROS or NF-kB was not sufficient to completely rescue the phenotypes associated with Rac1 loss. This is important in two ways; 1st: how robust the requirement for RAC1 is to intestinal tumor formation. As a key signaling node that integrates numerous downstream signaling pathways, the potential therapeutic benefits of targeting it should be high. Also, it would likely increase the range of tumors that would be sensitive to its inhibition and reduce the scope for drug resistance to develop. 2nd: it indicates that the RAC1 phenotype may also involve additional downstream pathways. In summary, downstream of WNT/MYC signaling after Apc loss that is crucial for hyperproliferation and hence tumorigenesis. Buczacki, S.J., Zecchini, H.I., Nicholson, A.M., Russell, R., Vermeulen, L., Kemp, R., and Winton, D.J. (2013). Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69. Cancer Genome Atlas Network. (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337. Zhou, C., Licciulli, S., Avila, J.L., Cho, M., Troutman, S., Jiang, P., Kossenkov, A.V., Showe, L.C., Liu, Q., Vachani, A., et al. (2013). The Rac1 splice form Rac1b promotes Kras-induced lung tumorigenesis. Oncogene 32, 903–909. ROS Production and NF-kB Activation Triggered by RAC1 Facilitate WNT-Driven Intestinal Stem Cell Proliferation and Colorectal Cancer Initiation. Myant et al., 2013. Cell Stem Cell. 2013.